THERAPY
Are you a #flozinator? SGLT2-inhibitors and chronic kidney disease

Dr. Anoushka Krishnan
Nephrologist, Royal Perth Hospital, Perth, Australia

Dr. Mythri Shankar
Assistant Professor of Nephrology,
Institute of Nephro-Urology,
Bengaluru, India

Dr. Nasim Wiegley
Assistant Professor of Medicine;
University of California,
Davis School of Medicine
Thanks to Twitter, the term flozinator has taken the nephrologist’s world by storm. Sodium-Glucose Co-transport 2 Inhibitors (SGLT2i) have come a long way in a short period of time; they are indeed revolutionizing the management of not only type 2 diabetes mellitus (T2DM) and diabetic chronic kidney disease (CKD) but also non-diabetic proteinuric CKD and heart failure.
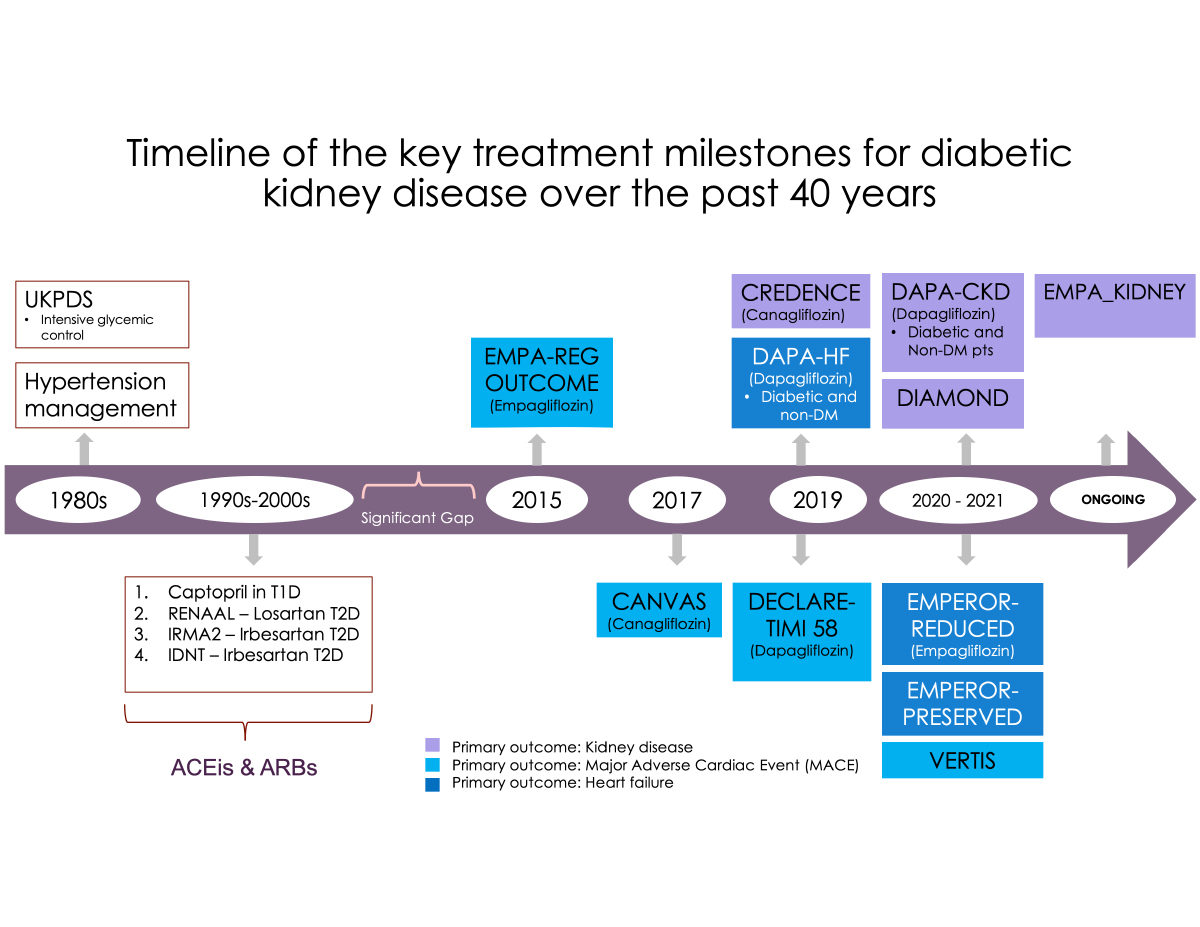
The chemical phloridzin was first isolated from the bark of apple trees in the 19th century by French scientists and was used in the treatment of malaria. It was later found to inhibit the reabsorption of glucose in the kidney. A couple of centuries later, the SGLT2 inhibitors were developed. The proximal tubules of the kidney house the SGLT2 protein, which aids the co-transport of glucose and sodium, contributing to nearly 90% of glucose reabsorption. SGLT2 inhibitors block this process resulting in glycosuria with reduction of serum glucose along with natriuresis and resultant improved tubuloglomerular feedback and reduced renal hyperfiltration. These drugs not only improve glycemic control but can also contribute to improved blood pressure, reduced albuminuria, improved lipid metabolism, lower serum uric acid levels, and modest weight loss, leading to positive outcomes in kidney and heart disease. Figure 1 demonstrates the timeline of various trials assessing the efficacy of SGLT2i.
Recent trial data (CREDENCE) showed a significant benefit of canagliflozin in patients with T2DM and CKD with estimated glomerular filtration rate (eGFR) 30 to <90 mL/min/1.73m2 and severe albuminuria (urine albumin: creatinine ratio [uACR] >33-565mg/mmol). Canagliflozin led to a 30% reduction in the composite risk for kidney failure (sustained eGFR < 15 mL/min/1.73m2 or treatment with dialysis or transplantation), a doubling of serum creatinine level, or death from kidney or cardiovascular causes (hazard ratio [HR]: 0.70, 95% confidence interval [CI]: 0.59-0.82). The relative risk of end-stage kidney disease was lower (by 32%, HR: 0.66, 95% CI: 0.53-0.81) in the canagliflozin cohort, as was the risk of acute myocardial infarction, stroke, or cardiovascular death (HR: 0.68, 95% CI: 0.67-0.95).
More recently, the DAPA-CKD trial, involving 4,304 participants, investigated the efficacy of dapagliflozin compared to placebo in patients with more advanced chronic kidney disease (eGFR of 25 to <75 ml/min/ 1.73m2) and albuminuria (uACR 22-565 mg/mmol [200-5000mg/gm]) with or without T2DM. This trial was stopped early due to the overwhelming efficacy of the study drug. Dapagliflozin slowed the rate of decline in eGFR, reduced progression to end-stage kidney disease, and mortality from kidney or cardiovascular death (HR: 0.61, 95% CI: 0.51-0.72), regardless of diabetes status. Cardio-renal benefits were observed across all stages of CKD (including stage 4 CKD with 624 patients in this cohort), all degrees of albuminuria, and HbA1C measurements (in diabetic individuals). It would be relevant to note that this study excluded patients with type 1 diabetes, polycystic kidney disease, CKD from lupus nephritis, ANCA vasculitis, or those on immunosuppressants six months prior to enrolment.
Additionally, a pre-specified analysis of the DAPA-CKD trial assessed 270 patients with IgA nephropathy, of whom 137 were randomized to dapagliflozin and 133 to placebo. The mean eGFR was 43.8 ml/min/1.73m2, and the mean albuminuria was 900mg/gm with a median follow-up of 2.1 years. The primary composite endpoint was a sustained decline in eGFR of 50% or more, end-stage kidney disease, or death from a kidney disease-related or cardiovascular causes. The primary outcome occurred in only six (4%) participants on dapagliflozin compared to 20 (15%) on placebo (hazard ratio: 0.29; 95% confidence interval, 0.12-0.73). Mean rates of eGFR decline with dapagliflozin and placebo were −3.5 and −4.7 mL/min/1.73m2/year, respectively. Dapagliflozin reduced the urinary albumin-to-creatinine ratio by 26% relative to placebo.
There were initial concerns about the risk of acute kidney injury (AKI) with these agents, with eGFR frequently dropping by 3-5ml/min after drug commencement, like that seen with RAASi. This was attributed to reversible intraglomerular hemodynamic effects. In fact, meta-analyses from several large-scale trials and propensity-matched studies showed that SGLT2i’s are associated with lower AKI risk (unlike the trade-off with RAASi’s, where long-term CKD protection is counter-balanced by increased AKI risk).
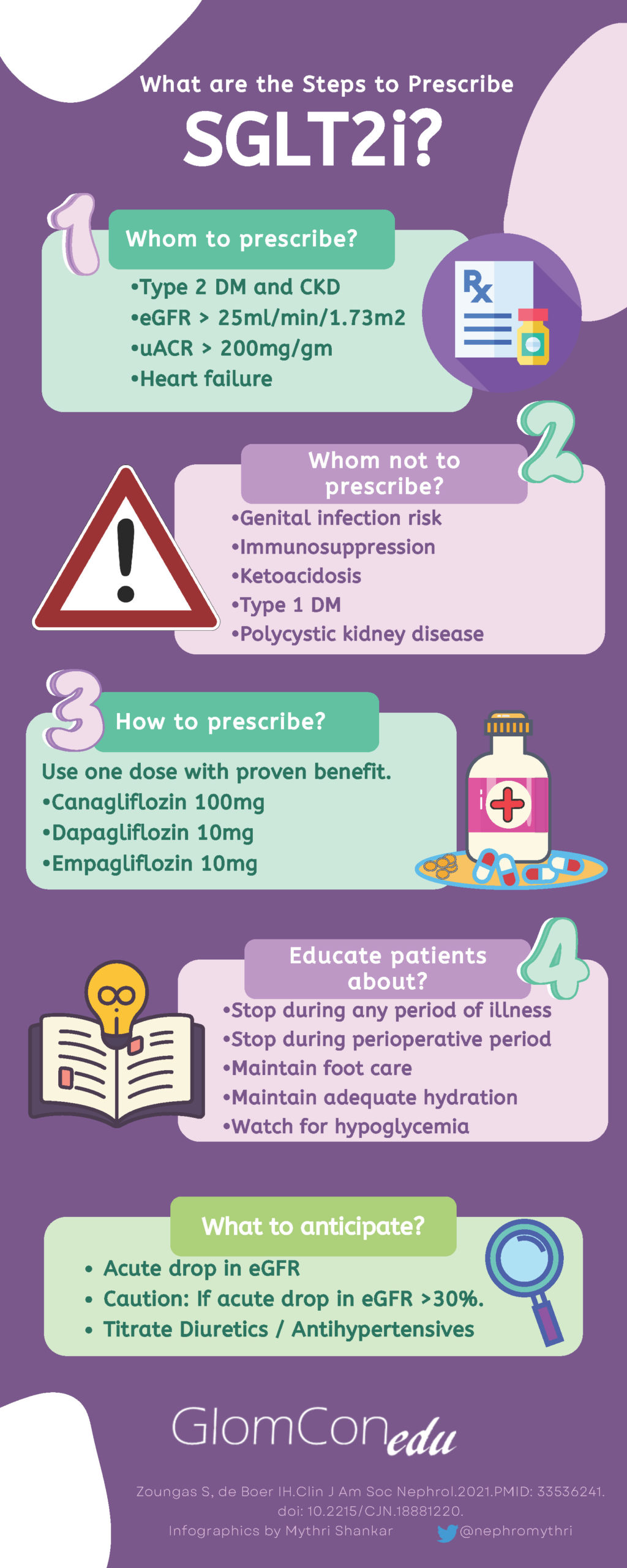
A thorough physical examination and assessment of volume status should be conducted prior to prescribing an SGLT2i. Diuretic and anti-hypertensive doses should be appropriately reduced or stopped in euvolemic patients to avoid volume depletion, and the commencement of these agents should be deferred in a hypovolemic/ hypotensive patient. In diabetic patients, the dose of certain oral hypoglycemic agents (such as sulfonylureas/ meglitinides) may be decreased by ~50% and/or insulin by 10-20% if fasting blood glucose levels are <6mmol/l (<110mg/dl) to reduce the risk of hypoglycemia. SGLT2i only lower plasma glucose levels by blocking the reabsorption of filtered glucose, which falls as plasma levels decline. Thus, hypoglycemia is uncommon with agents like metformin, glucagon-like peptide-1 receptor, dipeptidyl peptidase 4 inhibitors, and thiazolidinediones, which do not require dose adjustment on commencement of an SGLT2i. Figure 2 demonstrates a simple strategy for steps involved in the prescription of SGLT2i.
Patients should receive thorough counseling on the side effect profile of these agents, including genital mycotic infections, hypoglycemia in diabetics, and a small but significant risk of euglycemic diabetic ketoacidosis, particularly in long-standing T2DM with reduced beta-cell reserve. The initially proposed higher risk of urinary tract infections has now been deemed to be low, with a recent meta-analysis showing no significant increase in infection risk between SGLT2i and placebo. Patients should be encouraged to monitor their blood pressure, weight, and blood glucose levels at home when commencing an SGLT2i. Patients should also receive a clear ‘sick-day’ advice plan and should be advised to hold their SGLT2i if unwell, hypovolemic, hypotensive, or fasting for any procedures.
Conclusion:
SGLT2i have been proven to show enormous benefits in both cardiovascular disease and proteinuric kidney disease with or without diabetes. These drugs have a relatively low adverse effect profile, and the risks of acute kidney injury and urinary tract infections have now been demonstrated to be lower than initially expected. Prescriber hesitancy should be alleviated by using simple strategies of volume and laboratory assessment and adjusting doses of other antihypertensive or hypoglycemic agents to mitigate the potential adverse effects of these drugs. Future trials are eagerly awaited to assess the impact of SGLT2i agents on glomerular diseases, lower eGFR, non-proteinuric CKD, and other conditions.

Short Simple Effective