PROGNOSIS
International IgA Nephropathy Prediction tool: A step toward personalized medicine

By Dr Pallavi Prasad
Assistant Professor, Nephrology VMMC and Safdarjung Hospital
New Delhi, India
GlomCon Editors with significant contributions to the development of this article include: Sayali Thakare, Haresh Selvaskandan, Paolo Nikolai So, and Nasim Wiegley.
Disclaimer: No conflict of interest
Abstract
The International IgAN prediction tool was developed in 2018 to help predict the individual risk of disease progression in patients with primary IgAN. The tool uses clinical and histopathological markers at the time of biopsy to predict the risk of 50% decline in eGFR or kidney failure in patients with IgAN. It has been modified for use in the pediatric population and risk prediction at 1 and 2 years post kidney biopsy. Validation of the tool for various ethnicities has been done, but further studies are needed before it can be considered truly generalizable. Future modifications may also include biomarkers, which have been shown to improve the discriminatory power of the tool in small scale studies.
Introduction
Immunoglobulin A nephropathy (IgAN) is a common primary glomerular disease with a heterogeneous clinical presentation. The rate at which IgAN progresses to kidney failure is highly variable, with the risk of kidney failure at ten years varying between 5%–60%. The lifetime risk of kidney failure, however, appears to be much higher. A recent UK registry study including over 2000 adults and 100 children with biopsy-proven IgAN who had proteinuria > 0.5 grams/day found that over 80% developed kidney failure within 30 years unless the rate of estimated glomerular filtration rate (eGFR) decline was maintained at ≤ 1ml/min/1.73m². Crucially, progression to kidney failure within 10 years occurred even with low-grade proteinuria (< 0.88g/g).
A number of widely validated clinicopathological factors are known to influence the rate at which IgAN progresses to kidney failure. These include race, blood pressure, proteinuria, eGFR at the time of diagnosis, and histological findings (defined by the MEST-C score as detailed in Table 1). On their own, however, these estimate the risk of kidney failure at a population-wide level and provide little insight into the risk of progression of an individual patient. Individual risk prediction not only supports patient counseling and risk stratification but, in the future, may also help formulate individualized management plans. The International IgA Nephropathy prediction tool was developed to directly address some of these issues.
Table 1. The Oxford MEST-C SCORE
Adapted from: Cattran et al and Trimarchi et al.
The Original International IgAN Prediction Tool ( IIgANPT)
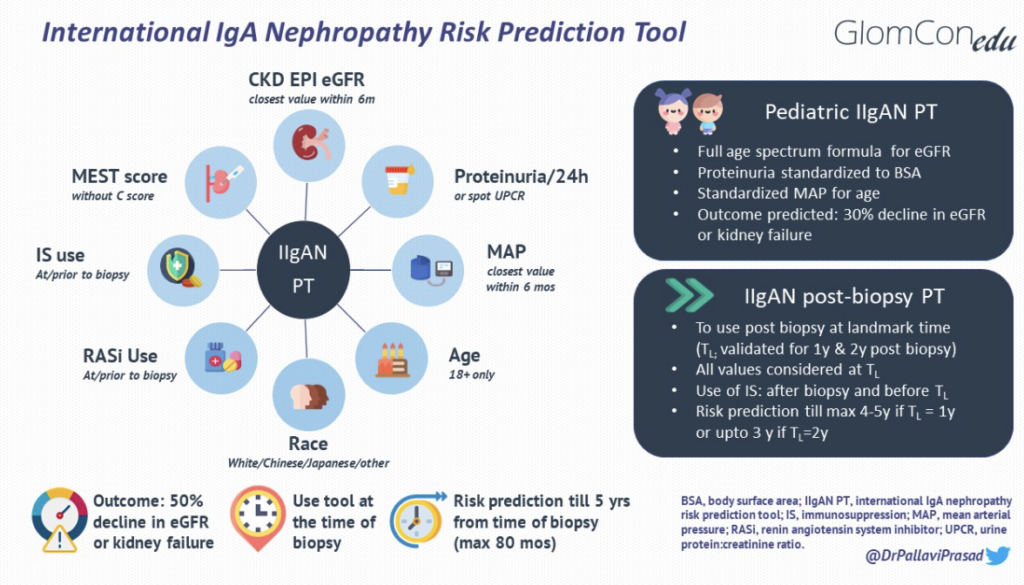
The IIgANPT integrates validated clinicopathological prognostic factors with treatments received at diagnosis to produce an individualized risk of disease progression (Figure 1). The model was derived from a multiethnic cohort of patients with biopsy-proven primary IgAN (n=2781 for derivation and n=1146 for external validation). Data were derived from established IgAN-related population studies, including cohorts from Europe, China, Japan, North and South America. Using data from the derivation cohort, three models were developed to predict a primary composite outcome of a 50% decline in eGFR or kidney failure (defined as eGFR <15 ml/min/1.73m2 by CKD EPI, dialysis or transplantation): the clinical, limited and full model. The clinical model incorporated known clinical predictors of outcomes [eGFR, proteinuria and mean arterial pressure (MAP)], and the limited model incorporated the MEST score into the clinical model. The full model, incorporated additional predictors of progression, including age, sex, race, crescents, body mass index (BMI), renin-angiotensin (RAS) blocker use at biopsy, immunosuppression at biopsy, and interaction terms between age and eGFR and between proteinuria and each of MAP, sex, RAS inhibition use at biopsy, and MEST. Race in the full model was specified as Chinese, Japanese, or White (since most of the derivation cohort hailed from these ethnicities). A full model without race was also developed. The two full models were found to have better discrimination, calibration and risk reclassification for predicting the primary outcome as compared to the clinical and limited models.
The tool was externally validated in a cohort of 1146 patients and found to have good discrimination ( C statistic 0.82; 95% CI, 0.81-0.83 with race/ethnicity; 0.81; 95% CI, 0.80-0.82 without race/ethnicity) and model fit R2D (both 35.3%) with excellent calibration as compared to the derivation cohort in predicting the composite outcome. The model is recommended for risk prediction at five years post-biopsy but can be used to predict risk up to seven years; five and seven-year timepoints were the 50th and 75th percentiles of follow-up duration in the derivation cohort.
The IIgANPT is yet to be validated as a tool for guiding treatment decisions, although it has been shown to predict the risk of disease progression in an individual patient more accurately than proteinuria alone. Nevertheless, The Kidney Disease Improving Global Outcomes (KDIGO) Management of Glomerular Diseases 2021 guidelines acknowledge its value in supporting patient counseling and facilitating shared decision-making and encourage the use of the IIgANPT for these purposes as a practice point (Practice Point 2.2.1)

Figure 1. IgAN risk prediction tools and its modifications
Crescents and the IIgANPT
It is now clear that the presence of crescents in IgAN is a predictor of poor outcomes in adults and in children. Crescents were adopted in the Oxford classification of IgA nephropathy in 2016 after the working group found that the percentage of glomeruli containing cellular or fibrocellular crescents was an independent risk factor for predicting a composite endpoint of 50% decline in eGFR or kidney failure (derived from a multi-ethnic cohort, n = 3096). Crescents correlated with the use of immunosuppression after biopsy; however, the risk of the composite outcome remained statistically significant in those with >25% glomerular crescents, regardless of immunosuppression.
The absence of crescents as a variable in the IIgANPT stems from incomplete data being available at the time the model was derived and validated. Crescents were initially incorporated into the model as either being present or absent, in contrast to the three levels assigned in the MEST-C score (C0 = 0% glomeruli with crescents, C1 <25% crescents and C2 ≥25% crescents). These granular data were not available for all cohorts, and when incorporated on the basis of presence alone, crescents strongly correlated with race ( Japanese > Chinese > White), which was an independent risk factor for risk prediction in the model. In the model without race, crescents did not satisfy the criterion for selection in the prediction model. When the variable for the use of immunosuppression was combined with the presence of crescents, it was found to satisfy the criterion for selection in the prediction model, but the use of immunosuppression post-biopsy on its own did not satisfy the primary criterion for inclusion as a predictor variable. For these reasons, crescents were excluded from the current IIgANPT.
Validation of the Tool Across Different Time Points
For decades, the only treatment options available for IgAN were RAS inhibition or, controversially, immunosuppression. These measures were often initiated after diagnosis and are expected to modify the percentage risk of progression to kidney failure. The original tool consistently underestimated the risk of disease progression when used at one or two years post-biopsy, perhaps due to the evolution of histology over time or due to a shift of time lag bias of eGFR by as many years. To account for changes over time, the IIgANPT was modified and externally validated for use one and two years after the biopsy. The updated tool incorporates the same clinicopathological parameters as used in the original tool but is recorded at the landmark time post-biopsy to predict the risk of the primary outcome ( e.g., proteinuria at one year for risk prediction at one year post-biopsy).
Notably, Japanese race and E1 on tissue histology had a higher hazard ratio for the composite endpoint, while eGFR, T1 and T2 lesions on biopsy had a lower hazard ratio compared to the original tool. The use of immunosuppression post-biopsy was found to be more frequent in patients with E1 lesions and Japanese race.
Although the same cohorts as used in the original tool were employed for derivation and validation of the updated tool, sufficient follow-up data was present in only one of the two Japanese cohorts. Further validation studies among Japanese and other ethnicities are needed to consider this model to be truly generalizable. The tool is most accurate when used one year post-biopsy. The median follow-up after the second year landmark time was 3.6 years, and hence risk calibration when the post-biopsy model was used at the second year (landmark time), especially in high-risk patients (risk of primary outcome >30%), may not be as accurate as when used at one year post-biopsy.
Validation of the tool for pediatric IgAN
In 2021, the IIgANPT was updated for use in children (age <18 years) with data collected from a multiethnic cohort recruited from China, Japan, Russia, Europe and North America. eGFR trajectory in children and adolescents with IgAN varies from that in adults – an initial rise in GFR is observed until about 18 years of age, followed by a linear fall (corresponding to the trajectory seen in adults). Due to this unique feature in children, the original prediction tool overestimated the risk in children.
Due to the unique trajectory of eGFR in the pediatric cohort, the composite primary endpoint was achieved in only a small percentage of those included (52/1060= 4.9%), and hence, a secondary outcome of 30% decline in eGFR or kidney failure was used for the prediction model in children. The survival curves also showed that the risk of a 30% decline in eGFR in children was analogous to a 50% decline in eGFR in adults. The pediatric tool is therefore used at the time of kidney biopsy for prediction of risk of 30% decline in eGFR or kidney failure over a maximum of 80 months post-biopsy, with similar factors as used in the adult tool, except for a few modifications made for use in the pediatric population. These changes include a change in the primary composite endpoint(as already mentioned earlier), using a full age spectrum formula for eGFR, body surface area normalized proteinuria quantification, and MAP standardized for age (summarized in Figure 1). Other iterations of the IIgANPT have not been validated in pediatric cohorts to date.
The characteristics of the tools are summarized in Table 2.
Table 2. IIgAN prediction tool- derivation studies and characteristics
Abbreviations: eGFR: estimated glomerular filtration rate, IIgAN PT: International IgAN prediction tool, KDIGO: Kidney Disease Improving Global Outcomes, miR: micro-RNA.
Validation of risk prediction tools across various cohorts & ethnicities
The IIgANPT has been externally validated in different ethnicities (Table 3) with varying outcomes. External validation in a Norwegian, a Chinese and another Chinese-Argentenian cohort have been published, and the models were found to have good discrimination and model fit. However, the risk probability over 3 years was overestimated in the full model (with race) in the Chinese-Argentenian cohort. Among patients from India, the tool had reasonable discrimination but underestimated the risk of progression across all risk groups. A validation study in a Korean cohort was also found to underestimate risk, more so in the model without race. The underestimation of risk in Indian and Korean cohorts further highlights the variability in risk progression in various ethnicities and possibly also the use of different management practices in different areas of the world. The tool may need further modification to be able to predict outcomes in ethnicities not adequately represented in the derivation or validation cohorts. In an increasingly multicultural world, ethnicity is often difficult to define, and categorization of risk progression on the basis of ethnicity alone should probably be replaced by categorization based on genetic makeup. A genetic risk score has recently been proposed for identifying patients prone to rapid progression of kidney disease. Also, environmental influences may also play a role in disease progression and need to be studied in more detail in the future.
There is a single validation study of the pediatric tool in Chinese population (Table 3) , and the post-biopsy tool has not yet been externally validated in other studies.
Table 3. Validation studies of IIgAN prediction tool (adult and pediatric)
Abbreviations: AUC: Area under Curve, IDI: Integrated Discrimination Improvement, NA: not available, NRI: Net Reclassification Improvement.
Potential uses of the tool
The IIgANPT can be used for risk stratification and prognostication of patients. It can not be used in its current form for treatment decisions.
Currently, most trial recruitment is based on eGFR, proteinuria and histopathological parameters as discrete and independent criteria to attempt to capture those with potentially reversible disease at greatest risk of progression. A complex interplay of various factors, some as yet unidentified, is involved in disease progression, as was seen in the derivation of the IIgAN PT. Although it is not approved in its current form for decision-making regarding immunosuppression, the tool may perform better than proteinuria for treatment allocation and would allow integration of risk factors to better capture those at the highest risk of progression for clinical trials in the future. However, underestimation or overestimation of risk in populations that were not well represented in the original cohorts may lead to recruitment bias. Incorporation of the tool in the trial recruitment process must be done carefully and with a conscious effort to prevent inequities in allocation or trial recruitment based on ethnicity.
A caveat to the use of this tool for therapeutic decision-making is the high risk of progression conveyed by both markers of fibrosis and activity. Although both of these may point towards a higher risk of progression, the treatment decision may be completely divergent in patients with fibrosis versus those with active lesions. It can also be modified to be used as a surrogate marker of response to therapy with a decrease in predicted risk post-treatment, signifying an improvement.
Future directions
Research in the field of IgAN is evolving rapidly, with new drugs being tested in both the disease modifying and enhanced supportive care domains. Drug efficacy trials in IgAN should ideally be able to demonstrate a change in the rate of loss of kidney function in patients treated with a drug. This often led to prolonged trials since GFR decline in IgAN may take years to develop. A Kidney Health Initiative project workgroup was established to look for surrogate endpoints for trials of IgAN. The workgroup found an association between treatment effects on percent reduction of proteinuria and treatment effects on a composite of time to doubling of serum creatinine, kidney failure, or death. Proteinuria reduction was hence established as a reasonably likely surrogate endpoint for a treatment’s effect on progression to kidney failure in IgAN. This facilitated the use of this endpoint for accelerated drug approvals by US FDA with the requirement of post marketing confirmatory endpoints to establish drug efficacy in IgAN.
With the acceptance of proteinuria as a surrogate endpoint in trials of IgAN, many new drugs may soon become a part of standard of care in IgAN. Recently, sparsentan (dual endothelin and angiotensin receptor blocker) and oral budesonide (targeted release formulation) have received accelerated approval for use in IgAN whereas iptacopan (small molecule factor B inhibitor) has shown results which may lead to its accelerated approval. Considering each could decrease rate of disease progression in IgAN, and maybe used in combination therapy in the future, the tool will need modifications incorporating their use in the therapeutic armamentarium of IgAN.
Combining the tool with biomarkers, including microRNA expression, has been shown to improve the prediction performance of the tool, although this needs to be validated in larger cohorts. Although the current tool will probably always be useful considering its practical applicability in all, settings, a modified tool combined with biomarkers may be useful in trial recruitments and research settings to identify subsets of patients who benefit most from a particular therapy.
Conclusion
The IgAN PT combines histological and clinical parameters in adults and children to estimate the risk of progression of disease in IgAN. It is a step towards personalized medicine in IgAN, since the estimation of risk can form the basis of important therapeutic decisions for individual patients and serve as a useful parameter for designing trials with the barrage of novel drugs purported to be useful in this disease. The tool needs validation across various ethnicities and possibly a modification of version to incorporate treatment-responsive versus treatment-resistant lesions before we use it to guide therapeutic decision-making.
