DISEASE MANAGEMENT
Management of children with steroid-resistant nephrotic syndrome (SRNS)

By Dr. Hamsa V, MD, DM (Ped Nephro)
Asst Professor – Paediatric Nephrology
Division of Paediatrics, Ramaiah Medical College Hospital and Ramaiah Memorial Hospital
Bengaluru, India
Nephrotic syndrome (NS) is a common problem, with a prevalence of about 1–3 per 100,000 children younger than 16 years of age. Most patients respond to the standard dose of oral prednisolone, and those who do not respond are presumed to have steroid-resistant nephrotic syndrome (SRNS) (2). Despite underlying histopathology, response to steroids is the most important prognostic factor in childhood NS.
Around 10-30% of children with SRNS have genetic etiology with mutations in genes encoding for the podocyte cytoskeleton. The rest are presumed to have circulating factors in the plasma. In most patients, renal histology of SRNS may show focal segmental glomerulosclerosis, minimal change disease, or diffuse mesangial sclerosis. The heterogeneous etiology, significant side effects related to further immunosuppression, risk of progression to chronic kidney disease, and post-transplantation recurrence make identification and optimal management of this condition important.
Evidence-based guidelines for diagnosis and management of SRNS are provided by KDIGO (Kidney Disease Improving Global Outcomes) and IPNA (International Pediatric Nephrology Association).
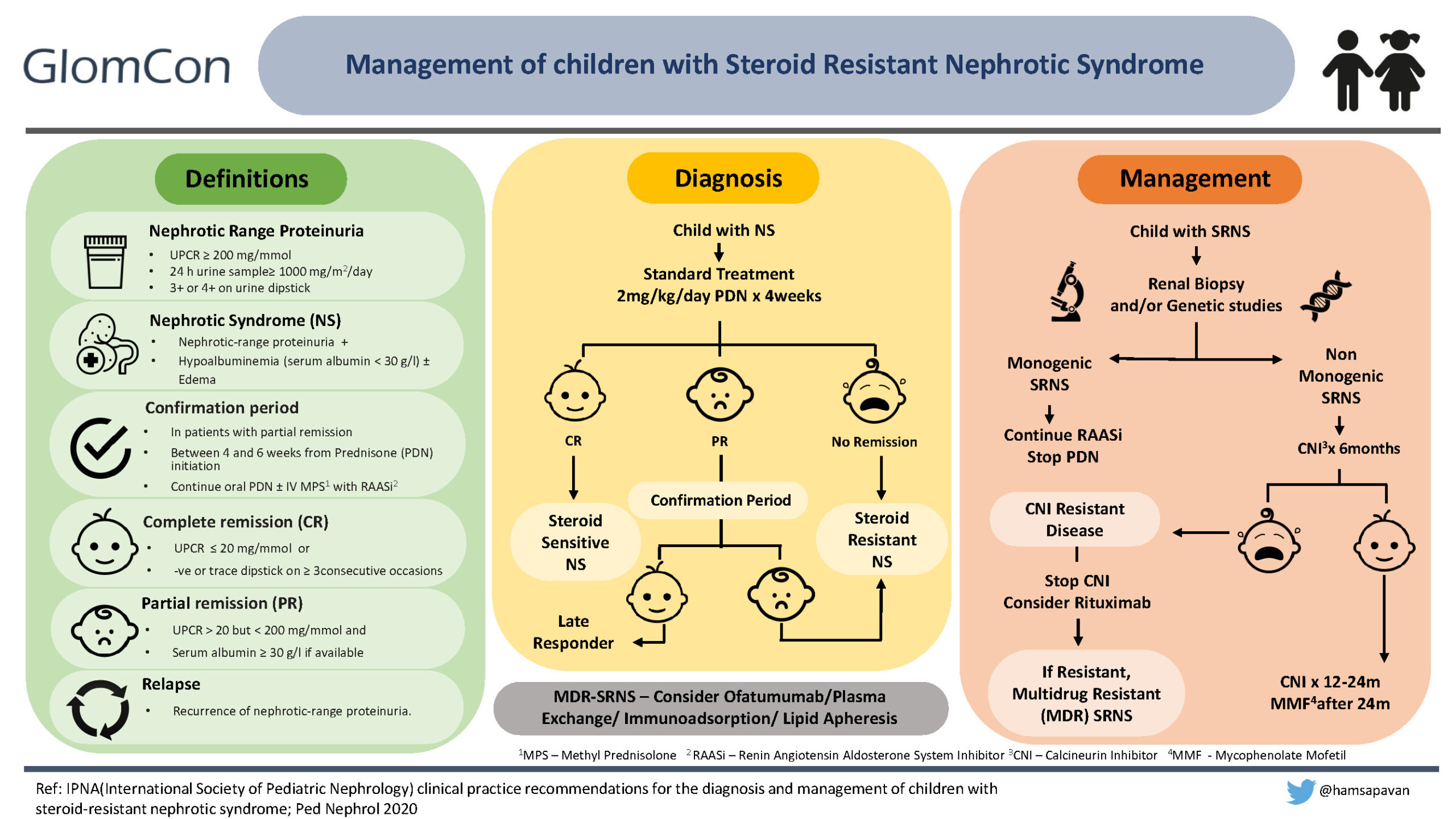
Definitions in children with Nephrotic syndrome
Nephrotic syndrome in children is defined as nephrotic range proteinuria [urine protein to creatinine ratio (UPCR) ≥ 200 mg/mmol (2 mg/mg) or 24 h urine protein ≥ 1gm/m2/day equivalent to 3+ or 4+ by urine dipstick], hypoalbuminemia with or without the presence of edema.
Children presenting with NS are treated with standard treatment, which includes oral prednisolone (PDN) at 60mg/m2/day or 2mg/kg/day for 4-6 weeks, followed by 40mg/m2 or 1.5mg/kg on alternate days for another 4-6 weeks. If the child achieves complete remission (defined as UPCR ≤ 20 mg/mmol (0.2 mg/mg) or negative or trace dipstick on ≥3 consecutive occasions) within the first four weeks of daily therapy, it confirms steroid-sensitive NS (6).
At the end of 4 weeks, a small percentage of children may achieve only partial remission (UPCR > 20 mg/mmol (0.2mg/mg) but < 200 mg/mmol (2mg/mg) and serum albumin ≥ 3g/dl). These children are continued on oral prednisolone with the addition of renin-angiotensin-aldosterone inhibitors with/without IV Methylprednisolone for the next 2 weeks, and this is defined as the confirmation period. If the child achieves complete remission within this period, he/she is a ‘late’ responder. If the child doesn’t achieve remission despite 4 weeks of standard therapy or continues to be in partial remission even after the confirmation period, he/she is labeled as steroid-resistant NS (SRNS).
Diagnostic workup of a child with SRNS
- History: A detailed family history and careful physical examination of the patient for renal and extrarenal manifestations of the disease should be obtained.
- Assessing for secondary causes: Secondary causes include infections (hepatitis B & C, Human Immunodeficiency Virus, hepatitis B, malaria, parvovirus B19) and autoimmune disorders (systemic lupus erythematosus, IgA nephropathy, and immune-complex mediated glomerular disorders). Testing includes:
- Blood tests to rule out immunological or infectious causes of SRNS and to evaluate the estimated GFR.
- The child should at least have a single quantification of proteinuria by UPCR in either a first morning (AM) urine or 24-hour urine sample prior to labeling SRNS and/or starting alternative immunosuppression.
- A renal biopsy should be performed in all children with SRNS, ideally during the confirmation period, to determine the underlying pathology.
- Genetic testing (Next Generation Sequencing Panel including all known SRNS genes, unless the clinical phenotype suggests a specific phenotype), if available, should be performed in all children with SRNS, ideally within the confirmation period. Genetic testing in SRNS children will help diagnose, identify treatable causes (e.g., CoQ deficiency), avoid unnecessary immunosuppression, and allow genetic counseling of the family, including prenatal diagnosis and risk of post-transplantation recurrence. Notably, Immunosuppression is not indicated in children with evidence of a monogenic form of SRNS.
Treatment recommendations in SRNS
- Calcineurin Inhibitor (CNI): cyclosporine or tacrolimus should be started as first-line immunosuppressive (IS) therapy. Table 2 demonstrates dosing and target trough levels of CNIs.
- Renin-angiotensin-aldosterone inhibitors (RAASi): Either angiotensin converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB) are recommended.
- Steroid therapy: oral PDN therapy should be tapered and stopped over 6 months.
- Cyclophosphamide (CYC): oral or intravenous CYC can be used with/without high-dose steroids when CNI is unavailable/unaffordable.
Table 2. CNI schedule and monitoring
| CNI | Dose | Target trough level |
|---|---|---|
| Tacrolimus | 0.1-0.2 mg/kg/day in 2 divided doses (max 5mg/day) | 4-8ng/ml |
| Cyclosporine | 3-5 mg/kg/day in 2 divided doses (max 250mg/day) | 80 -120ng/ml |
Response to CNIs:
Response to CNI is determined after a minimum treatment period of 6 months and cessation is considered if partial remission is not achieved at 6 months.
- If partial remission is achieved, CNI should be continued at the same dose for 12 months.
- If complete remission is achieved, CNIs should be discontinued after 12-24 months with transition to mycophenolate (1200mg/m2/day).
CNI resistant SRNS:
The child with SRNS not achieving even partial remission after 6 months of CNI treatment at adequate doses and/or levels is labeled as CNI-resistant SRNS.
- Children not achieving partial remission with CNI should be approached for participation in a clinical trial or alternatively, trial rituximab (two doses at 375mg/m2, two weeks apart to target CD19 count <1%).
- Children are labeled as multi-drug-resistant (MDR) SRNS if they fail to achieve CR after 12 months of two mechanistically different immunosuppressive drugs (including CNI).
- Ofatumumab, plasma exchange, immunoadsorption, or lipid apheresis may be considered in patients with MDR SRNS.
Supportive management:
Additionally, supportive management for all patients includes normal sodium, fluid, and protein intake, diuretics to manage edema, albumin infusion for refractory edema or symptomatic hypovolemia, Pneumocystis prophylaxis with rituximab use, and avoiding live vaccines while on immunosuppression.
Conflict of interest: None

Saludos colega, soy el Dr Daniel, nefrólogo pediátrico del Hospital Pediátrico José Luis Miranda de Villa Clara, Cuba. Me parece bien su actualización, me gustaría comentarle que en nuestro país no usamos de primera línea los anticalcineurinicos debido a que no podemos determinar su cuantía en sangre, y de esta menero no podemos saber la nefrotoxicidad, tengo muchos pacientes con síndrome nefrótico multirresitente (SRNS) que han transitado por esteroides, ciclosporina, algunos con pulsos de metil-prednisolona y otros que actualmente usan Mofetil Micofenolato.