DIAGNOSTIC
Pros and Cons of Serology-Based Testing for Primary Membranous Nephropathy

By Dr. Fatema Zaidi
University of Toronto
Toronto, Canada
GlomCon Editors with significant contributions to the development of this article: Vinay Srinivasan, Orhan Efe, Paolo So, and Nasim Wiegley
Abstract
Primary membranous nephropathy is a result of autoantibodies against glycoproteins in the glomerulus. Most commonly, the antibodies target PLA2R, a type 1 transmembrane glycoprotein expressed in the podocytes. Serological quantification of PLA2R antibodies can predict remission, relapse, and response to treatment. The latest KDIGO guidelines have introduced a serology-based approach to the diagnosis of primary membranous nephropathy while forgoing kidney biopsy in patients with preserved kidney function. This article reviews the pros and cons of this approach.
Introduction
Membranous nephropathy (MN) is the most common cause of nephrotic syndrome in non-diabetic adults. Primary membranous nephropathy is an autoimmune condition, with up to 70% of cases caused by anti-phospholipase A2 receptor (PLA2R) circulating autoantibodies and 3% by thrombospondin type-1 domain-containing 7A (THSD7A). These autoantibodies target the podocyte, inducing immune complex deposit formation at the basement membrane and resulting in proteinuria. Anti-PLA2R autoantibodies have not been recognized in other glomerular diseases, resulting in a specificity of close to 100% for membranous nephropathy.
Anti-PLA2R Autoantibodies
In 2009, the PLA2R protein was discovered and has since revolutionized the arena of primary MN. It is a 185 kilodalton (kDA) type 1 transmembrane glycoprotein found in the glomerulus. This protein is not only expressed on podocytes but also on neutrophils, pulmonary macrophages, airway submucosal epithelium and airway columnar epithelial cells. This is underscored by the fact that a higher degree of exposure to air pollution has been associated with the development of MN.
Kidney biopsy has been the gold standard for establishing a diagnosis of membranous nephropathy. However, with the introduction of highly specific autoantibodies, the role of biopsy becomes questionable. The latest KDIGO guidelines have introduced a serology-based approach to the diagnosis of primary membranous nephropathy in patients with preserved kidney function and a typical clinical presentation.
Diagnostic and Prognostic Utility of Serum anti-PLA2R Antibody in MN
Anti-PLA2R levels fluctuate with disease activity. Although proteinuria is not a reliable marker of immunologic activity, it helps stratify patients into high, moderate, or low risk of progressive loss of kidney function. Low baseline anti-PLA2R and decreasing levels were associated with higher rates of remission, and patients with undetectable levels after therapy had a higher likelihood of staying in remission for five years. In comparison, patients with higher baseline antibody titers were noted to have a lower chance of spontaneous remission, lower response to immunosuppressive therapy and a higher risk of declining kidney function. Patients with higher antibody levels also had more relapses of proteinuria and were more likely to have disease recurrence after kidney transplantation.
Overall, PLA2R antibody levels can predict remission, relapse, and response to treatment. KDIGO guidelines also recommend further immunosuppressive therapies based on PLA2R presence or absence 6 months after therapy. This allows us to not only use serum PLA2R levels as a diagnostic tool but also enables us to prognosticate and guide management without the added risks of performing a kidney biopsy.
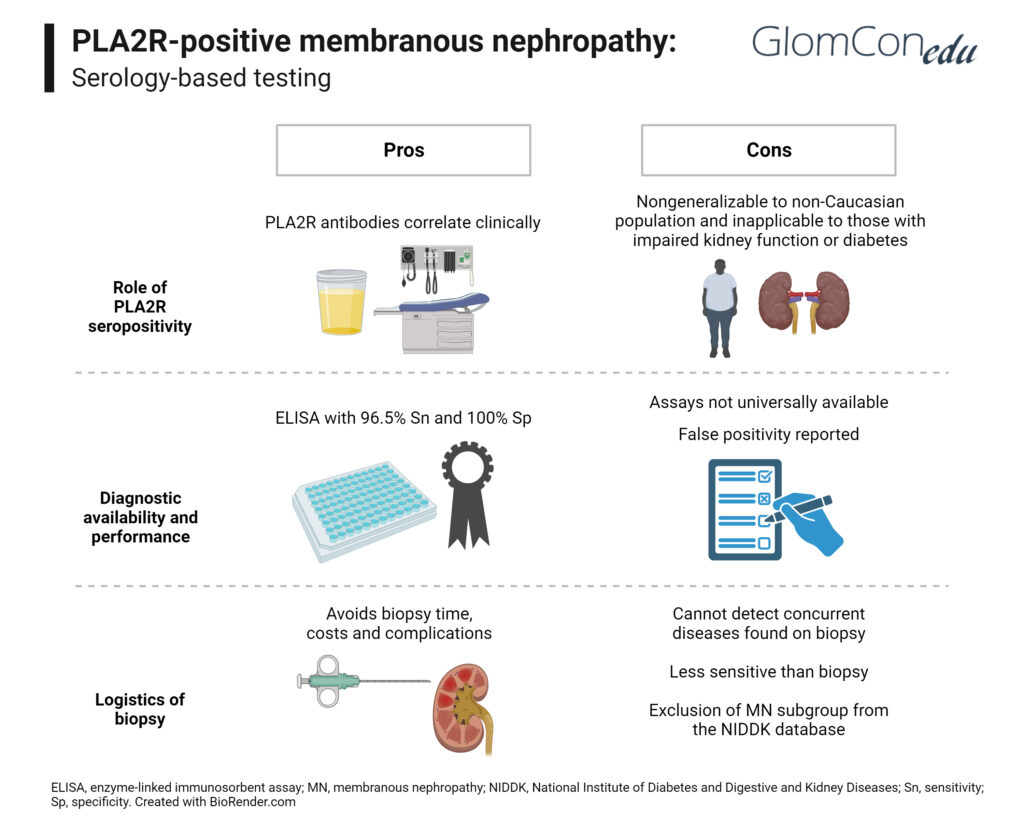
Pros of Using Serology-Based Diagnosis
PLA2R antibodies correlate clinically with disease activity. They can be used for diagnosis, prognostication and assessment of response to treatment. There are different serologic PLA2R assays, and a 2014 meta-analysis, evaluating 15 studies of over 2200 patients, found anti-PLA2R Ab to have a sensitivity of 78%, specificity of 99%, and positive likelihood ratio of 96% to detect idiopathic membranous nephropathy. Western blot is the most sensitive technique; however, it does not provide quantitative antibody levels and thus is not suitable for disease monitoring. Enzyme-linked immunosorbent assay (ELISA) is not as sensitive as western blot but provides quantitative antibody levels. Dahnrich et al. found ELISA to have a sensitivity of 96.5% at a set specificity of 99.9%. Lastly, cell-based assay indirect immunofluorescence (CBA IFA) is a method of high diagnostic accuracy and is a beneficial method to detect low levels of the antibody if ELISA is not conclusive.
Kidney biopsy carries a risk of severe complications. A meta-analysis of over 100,000 native kidney biopsies found that the rate of fatal complications ranged from 0.02-0.06% and grade 3 hemorrhage (radiologic, endoscopic, or elective operative intervention indicated) was 0.3%. The need for transfusion ranged from 0.9-1.6% and rate of interventions needed for bleeding was 0.3%. Complications were higher in hospitalized patients and those with acute kidney injury (AKI). Thus, serological diagnosis helps to avoid the risks associated with kidney biopsy.
Moreover, there are many comorbidities, such as uncorrectable bleeding diathesis, pregnancy, and solitary kidney, that increase the risk for complications; negating the need for biopsy in this population is even more advantageous. This is also beneficial since biopsy may not readily be available in remote areas. Serologic testing may be faster and more convenient if the institution has in-house ability to preform PLA2R testing.
It is also important to note that biopsy does not add to the prognostication of MN in patients with normal kidney function. Bobart et al. quantified the total renal chronicity score (TRCS) on all primary MN biopsies. Patients with an eGFR > 60 ml/min had low TRCS scores of 0-1, demonstrating that biopsy did not add to the prognostication of MN. They further showed that proteinuria did not relate to prognostication either; patients with TRCS of 0-1 and those with a TRCS of 3-4 had similar proteinuria ranges of 3 g to 21 g. They further concluded that the degree of interstitial fibrosis found on biopsy did not impact their response to therapy.
Cons of Using Serology-Based Diagnosis
In 2019, Bobart et al concluded that in those with normal kidney function and a negative work up for secondary causes or diabetes, a positive PLA2R would suffice for a diagnosis of MN, and biopsy would be unnecessary. However, there were some limitations in this study. First, it is important to note that the authors used either an ELISA >20 RU/mL or IFA as positive serology. However, there was a patient with ELISA <2 RU/mL but positive IFA and another with ELISA >20 RU/mL but negative IFA. Many labs only perform one of the two tests (either IFA or ELISA) and patients may therefore be misdiagnosed. Serologic assays are also relatively new and may not be available worldwide. Secondly, the majority of study patients were White men. Although this is expected to occur in a MN population, we must question if the data is generalizable to other populations. Lastly, these guidelines are not applicable to those with a decreased GFR of < 60 ml/min or with diabetes, thus excluding a significant number of patients.
It is also important to note that Weich et al. conducted a similar study in 2019 that examined the likelihood of a secondary diagnosis (besides MN) in those with preserved kidney function and positive PLA2R antibody. A total of 12/184 (6%) of patients had a secondary diagnosis and 2 of the 12 patients (1% of total patients) had IgA nephropathy. The study, however, does not elaborate whether discovery of a second diagnosis impacted management.
Primary MN can also be diagnosed by staining for the antigen by immunofluorescence or immunoperoxidase in the kidney tissue. This has a specificity of nearly 100%. Of note, in the landmark trial by Bobart in 2019, 26% of biopsies staining for PLA2R had negative serologies. Kidney biopsy is also a more sensitive tool for diagnosing PLA2R-related membranous nephropathy, secondary to rapid antibody clearance or the sink effect. In 2012, Hoxha et al. also described that the intensity of staining for PLA2R in the glomeruli may help discriminate between primary and secondary MN although the study had a relatively small sample size.
The most important concern is that false positives for anti-PLA2R serology may occur. Although case reports are limited, they show that patients (even with nephrotic syndrome) have tested positive for anti-PLA2R with ELISA assays (well above the defined cut-off points) but do not have findings of membranous nephropathy on biopsy potentially leading to unnecessary immunosuppressive therapy. In one specific case, serum PLA2R antibodies were tested via ELISA four times over 6 months, with values ranging from 120-258 RU/ml (cut off for positivity defined as 20 RU/ml), however extensive immunohistochemical analysis and repeat biopsies 1 month apart did not show evidence of membranous nephropathy.
Biopsy, and not serology, can also help us differentiate between primary and secondary MN. Subepithelial, intramembranous and mesangial deposits are commonly seen in secondary MN along with mesangial or endocapillary proliferation, full-house immunofluorescence, tubular basement membrane electron-dense deposits, or endothelial tubuloreticular inclusions, whereas isolated subepithelial deposits are mostly seen in primary MN. Furthermore, primary MN primarily stains for IgG4 whereas lupus membranous nephropathy predominantly stains for IgG1, IgG2, IgG3, and malignancy-associated MN stains for IgG1 and IgG2.
Withholding biopsies also removes the possibility of applying innovative techniques to biopsy slides. The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Kidney Precision Medicine Project aims to analyze multiple kidney biopsies to build a database to help develop targeted new therapeutics. By removing the need for biopsies in PLA2R-positive MN, we remove this subgroup from the project. Furthermore, an initial biopsy provides a baseline to compare against future biopsies if there are clinical or laboratory changes. If a biopsy is performed for AKI without a baseline biopsy present, it is difficult to discern whether the changes are due to a new or prior diagnosis.
The role of anti-PLA2R serology in secondary MN is also unclear. Qin et al. found anti-PLA2R antibodies in patients with secondary MN with underlying lupus, Hepatitis B and malignancy. Patients who were anti-PLA2R and Hepatitis B positive did not enter remission after antiviral therapy, and anti-PLA2R positive patients who underwent tumor resection still had persistence or recurrence of proteinuria. Interestingly, patients with positive PLA2R serologies and secondary causes had predominant IgG4 antibodies, which are typically found in primary and not secondary MN. In 2013, Larsen et al. examined the role of PLA2R in a population of secondary MN patients. Nearly 64% of patients with Hepatitis C, 3 of 4 patients with sarcoidosis, and 25% of patients with malignancy had positive PLA2R staining on biopsy. Again, all cases stained for IgG4. Therefore, the presence of anti-PLA2R antibodies does not negate the need to evaluate for secondary causes, as the two can coincide.
Conclusion
Anti-PLA2R levels are helpful in patients with preserved kidney function and no clear secondary cause; they provide a sensitive and specific method to diagnose, prognosticate and manage MN. With the discovery of newer antigens, we need to further evaluate the role of biopsy versus serology-based testing once these become readily available worldwide. While there are many advantages to avoiding kidney biopsy, it should still be considered on a case-by-case basis depending on the clinical context.
References
Bech AP, Hofstra JM, Brenchley PE, Wetzels JF. Association of anti-PLA(2)R antibodies with outcomes after immunosuppressive therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2014;9(8):1386–1392.
Beck L. H. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. New Engl J Med. 2009. 361, 11–21.
Bobart SA, De Vriese AS, Pawar AS, et al. Noninvasive diagnosis of primary membranous nephropathy using phospholipase A2 receptor antibodies. Kidney Int. 2019; 95:429–438.
Bobart SA, Heedeok H, Shahrzad T, An S. De Vriese,, Juan C Leon R, Sanjeev S, Ladan Z, Cristina A Gomez, Callen D. Giesen, Maria JS, Andrew S. Bomback, and Fernando C. Fervenza. Clin J Am Soc Nephrol. 2021 Dec; 16(12): 1833–1839.
Caza Tiffany et al. Discovery of Seven Novel Putative Antigens in Membranous Nephropathy and Membranous Lupus Nephritis Identified by Mass Spectrometry”. Kidney International, no. 3, Mar 2023.
Dahan K, Gillion V, Johanet C, Debiec H, Ronco P. The role of PLA2R antibody in treatment of membranous nephropathy. Kidney Int Rep. 2018;3(2):498–501.
Dahnrich C, Komorowski L, Probst C, Seitz-Polski B, Esnault V, et al. (2013) Development of a standardized ELISA for the determination of autoantibodies against human M-type phospholipase A2 receptor in primary membranous nephropathy. Clin Chim Acta 421: 213–218.
Dähnrich C, Saschenbrecker S, Gunnarsson I et al. Development of a standardized chemiluminescence immunoassay for the detection of autoantibodies against human M-type phospholipase A2 receptor in primary membranous nephropathy. Kidney Int Rep 2019; 5: 182–188.
De Vriese AS, Glassock RJ, Nath KA, Sethi S, Fervenza FC: A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol 28: 2017. 421–430.
Dong HR, Wang YY, Cheng XH, Wang GQ, Sun LJ, Cheng H, Chen YP. Retrospective study of phospholipase A2 receptor and IgG subclasses in glomerular deposits in Chinese patients with membranous nephropathy. PLoS One. 11: e0156263, 2016
Du Y, Li J, He F, Lv Y, Liu W, Wu P, Huang J, Wei S, Gao H: The diagnosis accuracy of PLA2R-AB in the diagnosis of idiopathic membranous nephropathy: A meta-analysis. PLoS One 9: e104936, 2014.
Markowitz GS. Membranous glomerulopathy: emphasis on secondary forms and disease variants. Adv Anat Pathol, 8 (2001), pp. 119-125
Hofstra JM, Beck LH Jr., Beck DM, Wetzels JF, Salant DJ: Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 6: 1286–1291, 2011.
Hoxha E, Harendza S, Pinnschmidt H, Panzer U, Stahl RA: PLA2R antibody levels and clinical outcome in patients with membranous nephropathy and non-nephrotic range proteinuria under treatment with inhibitors of the renin-angiotensin system. PLoS One 9: e110681, 2014
Hoxha Elion, Reinhard Linda, Castedello Thomas, Becker Juan. False positivity for PLA2R1 antibody measured by ELISA in a nephrotic patient with no membranous nephropathy. Kidney International (2023) 103, 411–41
Hoxha E., Harendza S, Zahner G, Panzer U, Steinmetz O, Fechner K, Helmchen U, Stahll R. An immunofluorescence test for phospholipase-A₂-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant 2011 Aug;26(8):2526-32
Hoxha E, Kneissler U, Stege G, Zahner G, Thiele I, Panzer U, Harendza S, Helmchen UM, Stahl RA. Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int. 2012;82(7):797–804.
Kanigicherla D, Gummadova J, McKenzie EA, Roberts SA, Harris S, Nikam M, Poulton K, McWilliam L, Short CD, Venning M, Brenchley PE. Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int. 2013;83(5):940–948.
Larsen C, Messias N, Silva F, Messias E, Walker P. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod Patahol. 2013 May;26(5):709-15.
Mahmud M, Pinnschmidt HO, Reinhard L, Harendza S, Wiech T, Stahl RAK, Hoxha E. Role of phospholipase A2 receptor 1 antibody level at diagnosis for long-term renal outcome in membranous nephropathy. PLoS One. 2019;14(9):e0221293.
Ohtani H, Wakui H, Komatsuda A, Okuyama S, Masai R, Maki N, Kigawa A, Sawada K, Imai H. Distribution of glomerular IgG subclass deposits in malignancy-associated membranous nephropathy. Nephrol Dial Transplant. 2004;19(3):574–579.
Poggio ED, McClelland RL, Blank KN, Hansen S, Bansal S, Bomback AS, Canetta PA, Khairallah P, Kiryluk K, Lecker SH, McMahon GM, Palevsky PM, Parikh S, Rosas SE, Tuttle K, Vazquez MA, Vijayan A, Rovin BH: Kidney Precision Medicine Project: Systematic review and meta-analysis of native kidney biopsy complications. Clin J Am Soc Nephrol 15: 1595–1602, 2020
Qin Weisong, Laurence H. Beck, Jr, and Zhihong Liu. Anti-Phospholipase A2 Receptor Antibody in Membranous Nephropathy. JAM soc nephrol 2011 June 22(6) 1137-1143
Ronco Pierre and Plaisier Emmanuelle. Time to Abandon kidney biopsy to diagnose membranous nephropathy?. Clin J Am Soc Nephrol. 2021 Dec; 16(12):1787-1789.
Rovin BH, Adler SG, Barratt J, Bridoux F, Burdge KA, Chan TM, et al. . KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int (2021).
Silliman CC, Moore EE, Zallen G, Gonzalez R, Johnson JL, Elzi DJ, Meng X, Hanasaki K, Ishizaki J, Arita H, Ao L, England KM, Banerjee A. Presence of the M-type sPLA(2) receptor on neutrophils and its role in elastase release and adhesion. Am J Physiol Cell Physiol. 2002;283(4):C1102–1113.
Svobodova B, Honsova E, Ronco P, Tesar V, Debiec H: Kidney biopsy is a sensitive tool for retrospective diagnosis of PLA2R-related membranous nephropathy. Nephrol Dial Transplant 28: 1839–1844, 2013
Von Haxthausen F, Reinhard L, Pinnschmidt HO, Rink M, Soave A, Hoxha E, Stahl RAK. Antigen-specific IgG subclasses in primary and malignancy-associated membranous nephropathy. Front Immunol. 2018;9:3035.
Tomas N, Tobias H, Hoxha E. Perspectives in membranous nephropathy. Cell Tissue Res. 2021; 385(2): 405–422.
Wiech T, Stahl RAK, Hoxha E. Diagnostic role of renal biopsy in PLA2R1-antibody-positive patients with nephrotic syndrome. Mod Pathol. 2019;32(9):1320–1328.
Xipell M, Rodas LM, Villarreal J, Molina A, Reinoso-Moreno J, Blasco M, Poch E, Diekmann F, Campistol JM, Quintana LF. The utility of phospholipase A2 receptor autoantibody in membranous nephropathy after kidney transplantation. Clin Kidney J. 2018;11(3):422–428.
Xu X, Wang G, Chen N, Lu T, Nie S, Xu G, Zhang P, Luo Y, Wang Y, Wang X, Schwartz J, Geng J, Hou FF. Long-term exposure to air pollution and increased risk of membranous nephropathy in China. J Am Soc Nephrol. 2016;27(12):3739–3746.