MINI REVIEW
Target Antigens and Noninvasive Markers in Membranous Nephropathy
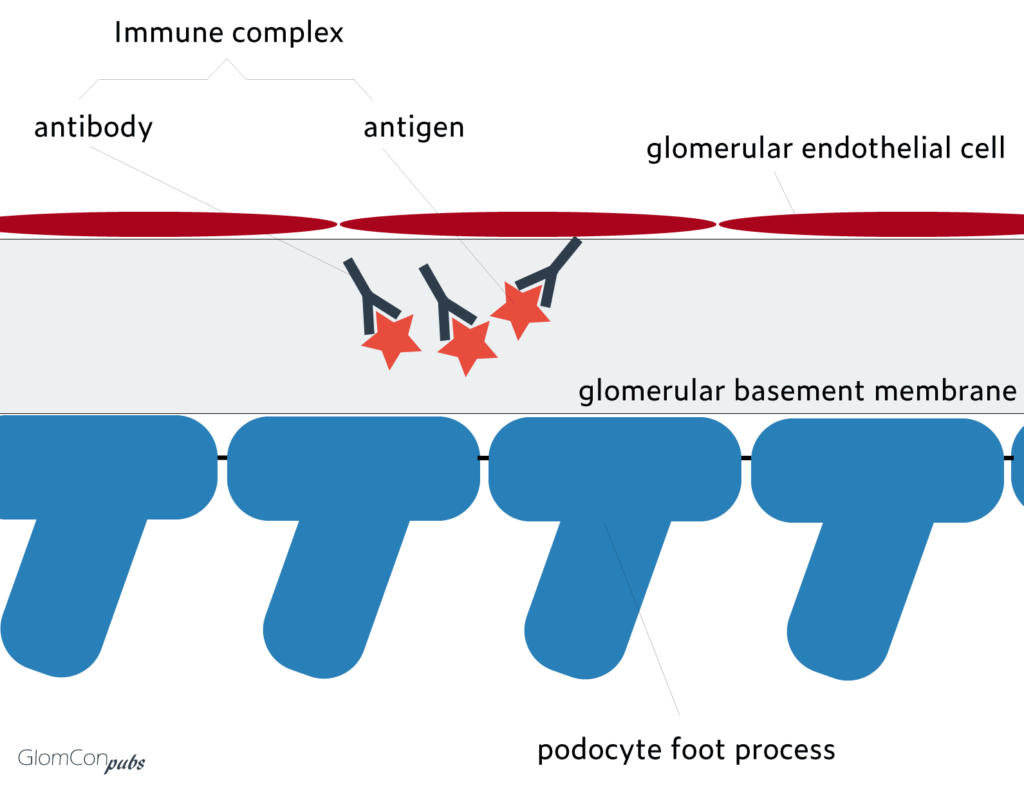
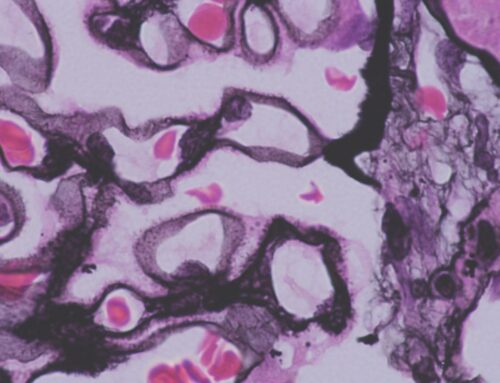
Membranous nephropathy (MN) is one of the most common causes of nephrotic syndrome in adult patients. Up to eighty percent are primary, and ~20% are due to secondary causes (HBV, autoimmune, malignancy, etc.). The characteristic pathological finding is intramembranous immune complex deposits at the glomerular basement membrane (GBM).

By Dr. Orhan Efe
Nephrology Fellow, PGY5, Brigham and Women’s / Massachusetts General Hospital Combined Nephrology Program, Boston, MA
In primary MN, antigen-antibody complexes develop in situ by targeting of podocyte antigens rather than trapping of circulating immune complexes. Target podocyte antigens remained unknown for many years until the last decade. The identification of target podocyte antigens and corresponding autoantibodies has enlightened the pathophysiology and changed clinical practice remarkably.
In 1959, Heymann and colleagues created the first animal model of membranous nephropathy by inducing an autoimmune reaction through intraperitoneal injection of kidney tissue suspension into rats. The antigen, against which the autoantibodies were formed, was identified to be megalin. Megalin is a member of LDL receptor superfamily. It is expressed on rat podocytes but not on human podocytes. The target podocyte antigen(s) in the human kidney remained unknown for many years. In 2002, Debiec and colleagues identified maternal alloantibodies that targeted fetal podocyte neutral endopeptidase and led to nephrosis in newborns. The formation of alloantibodies was due to a hereditary deficiency of neutral endopeptidase in the mother (similar to Rh intrapartum sensitization).
Although the discovery of fetal podocyte neutral endopeptidase as the target antigen in newborn nephrosis was a milestone in our understanding of the pathophysiology of MN, the main target in primary MN remained unknown. Finally, in 2009, Beck et al. discovered phospholipase A2 receptor (PLA2R, a transmembrane receptor expressed on the surface of human podocytes) as the target antigen in the majority of primary MN cases. Through elegant experimental design, Beck and colleagues analyzed healthy glomerular protein extract with Western blot and used sera of MN patients as the antibody source. A positive band in Western blot was identified, suggesting the presence of antibodies against an antigen within the glomerular protein extract. Through mass spectrometry, the antigen was identified to be PLA2R. Since its discovery, anti-PLA2R antibody assays have significantly changed our clinical practice.
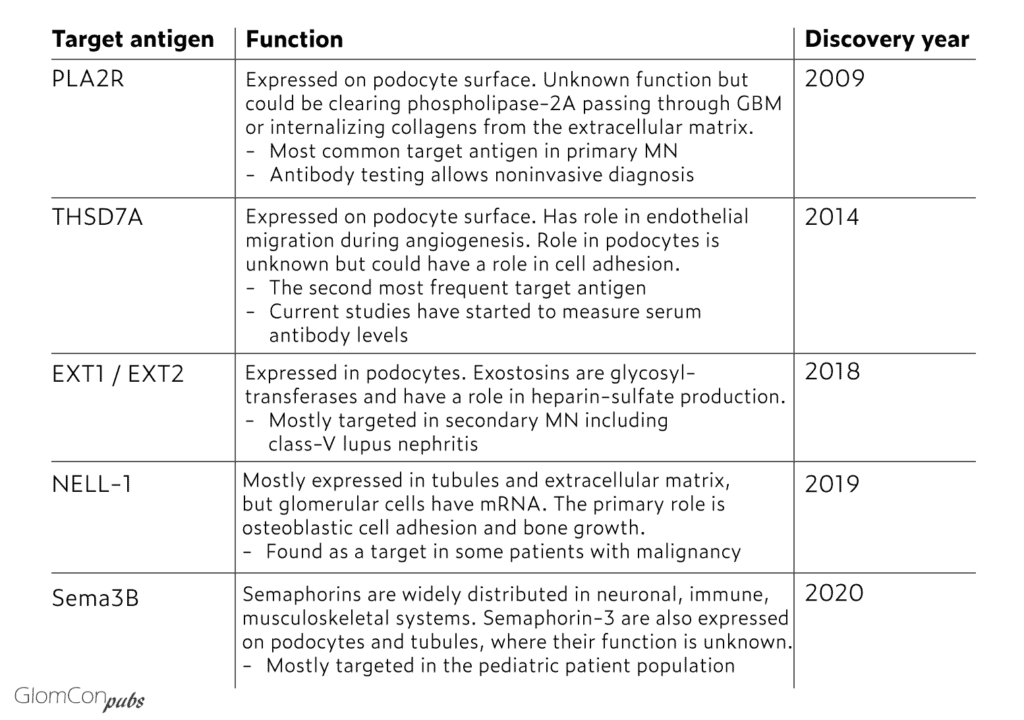
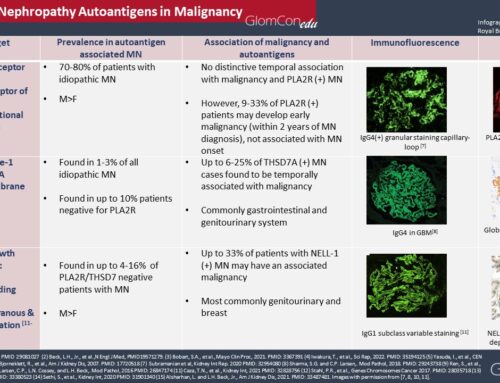
In 2014, following the discovery of PLA2R as the target antigen for most primary MN cases, Thomas et al. identified thrombospondin type 1 domain-containing 7A (THSD7A) as another target in PLA2R-negative primary MN patients. Furthermore, in 2018, Sethi et al. used laser microdissection and mass spectrometry to extract and analyze proteins from biopsy samples of PLAR2-negative primary and secondary MN patients. This approach identified Exostosin1/Exostosin 2 (EXT1/EXT2) as an additional target antigen in MN. Interestingly, 80% of EXT1/EXT2 positive cases had autoimmune features, including class-5 lupus nephritis. It was also positive in a few primary MN patients. In 2019, the same group identified NELL-1 as another target in double-negative patients (PLA2R and THSD7A negative). Notably, some of the NELL-1 positive patients were diagnosed with malignancies. In 2020, the same group again discovered targeting of semaphorin-3B (sema3B) in double negative primary MN patients and showed co-localization of IgG and sem3B in the GBM in 11 kidney biopsies. Most of those (8 patients) were in the pediatric age group. Currently, known target antigens collectively correspond to 80% of primary MN cases. Further targets are still to be identified. Known target antigens are listed in the table.
Diagnostic testing and clinical correlation
Anti-PLA2R antibody
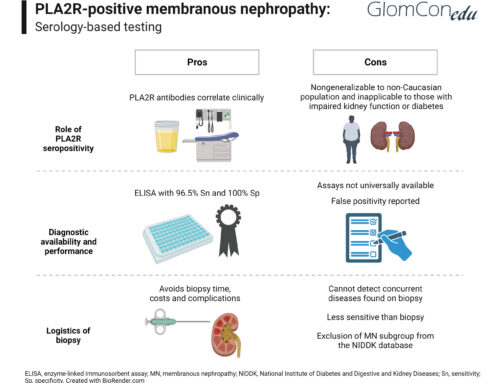
Although several autoantibodies have been discovered, only the anti-PLA2R antibody is in practical use in most centers. PLA2R is the target antigen in 70% of primary MN. Enzyme-linked immunosorbent assay (ELISA) and immunofluorescent assay (IFA) are two methods to analyze anti-PLA2R antibodies. ELISA is a semi-quantitative test and more commonly used than IFA. The sensitivity of ELISA is around 70%, and specificity is almost 100% in primary MN. A small minority of patients with secondary MN were found to have positive anti-PLA2R antibody titers, and these were most likely coincidental given IgG4 predominance in pathological staining, which is considered to be characteristic of primary MN (Qin et al., 2011). A prospective multicenter study has shown that PLA2R titers correlate with disease activity and clinical outcome. Among the patients treated with different immunosuppression regimens using rituximab, a calcineurin inhibitor, or alkylating agents, around 80% had a significant decline in anti-PLA2R antibody titers within three months. In the same study, higher baseline serum anti-PLA2R antibody titers correlated with more resistant disease. In summary, serum titers correlate with spontaneous remission, disease progression and relapse, proteinuria trajectory, and therapeutic response.
Anti-THSD7A antibody
Autoantibodies against THSD7A are also emerging as a biomarker. In 2019, Zaghrini and colleagues developed an ELISA assay to quantify anti-THSD7A antibody titers. They analyzed 1012 biopsy-proven MN patients from different cohorts and additional local patients and identified 49 THSD7A-positive MN patients. The ELISA testing showed good sensitivity and specificity since it correlated well with IFA, Western blot, and biopsy staining. Moreover, anti-THSD7A antibody titers correlated with treatment and disease activity. Antibody titers were significantly higher in patients with active disease compared to those in remission. Among the 12 patients who had available follow-up sera, anti-THSD7A antibody titers became either undetectable or decreased significantly with remission of disease after either conservative management or immunosuppressive treatment.
In summary:
The Identification of PLA2R as a target antigen has changed our clinical practice significantly. Some experts recommend forgoing kidney biopsy in patients with nephrotic syndrome who have an elevated serum anti-PLA2R antibody titer, a normal kidney function, a negative workup for secondary causes of MN, and no history of diabetes (Bobart et al., 2019). The serum anti-PLA2R antibody titers correlate with spontaneous remission, disease progression, relapse, degree of proteinuria, and therapeutic response. The discovery of target antigens has enlightened the pathophysiology of most primary MN presentations. The testing of antibodies against these proteins will continue to change the clinical practice. Antibody panels may allow noninvasive diagnosis, disease monitoring, and the discovery of new therapeutics if they can be validated to serve as surrogate endpoints in clinical trials.
References
- Heymann W, Hackel DB, Harwood S, Wilson SG, Hunter JL. Production of nephrotic syndrome in rats by Freund’s adjuvants and rat kidney suspensions. Proceedings of the Society for Experimental Biology and Medicine. 1959 Apr;100(4):660-4.
- Debiec H, Guigonis V, Mougenot B, Decobert F, Haymann JP, Bensman A, Deschênes G, Ronco PM. Antenatal membranous glomerulonephritis due to anti–neutral endopeptidase antibodies. New England Journal of Medicine. 2002 Jun 27;346(26):2053-60.
- Beck Jr LH, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. New England Journal of Medicine. 2009 Jul 2;361(1):11-21.
- Tomas NM, Beck Jr LH, Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, Dolla G, Hoxha E, Helmchen U, Dabert-Gay AS, Debayle D. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. New England Journal of Medicine. 2014 Dec 11;371(24):2277-87.
- Sethi S, Madden BJ, Debiec H, Charlesworth MC, Gross L, Ravindran A, Hummel AM, Specks U, Fervenza FC, Ronco P. Exostosin 1/exostosin 2–associated membranous nephropathy. Journal of the American Society of Nephrology. 2019 Jun 1;30(6):1123-36.
- Sethi S, Debiec H, Madden B, Charlesworth MC, Morelle J, Gross L, Ravindran A, Buob D, Jadoul M, Fervenza FC, Ronco P. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney international. 2020 Jan 1;97(1):163-74.
- Sethi S, Debiec H, Madden B, Vivarelli M, Charlesworth MC, Ravindran A, Gross L, Ulinski T, Buob D, Tran CL, Emma F. Semaphorin 3B-associated membranous nephropathy is a distinct type of disease predominantly present in pediatric patients. Kidney International. 2020 Jun 11.
- Zaghrini C, Seitz-Polski B, Justino J, Dolla G, Payré C, Jourde-Chiche N, Van de Logt AE, Booth C, Rigby E, Lonnbro-Widgren J, Nystrom J. Novel ELISA for thrombospondin type 1 domain-containing 7A autoantibodies in membranous nephropathy. Kidney international. 2019 Mar 1;95(3):666-79.
- Hoxha E, Thiele I, Zahner G, Panzer U, Harendza S, Stahl RA. Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. Journal of the American Society of Nephrology. 2014 Jun 1;25(6):1357-66.
- Bobart SA, Fervenza FC. Kidney biopsy is required for nephrotic syndrome with PLA2R+ and normal kidney function: The CON view. Kidney360. 2020 Jan 1.
- Bobart SA, De Vriese AS, Pawar AS, Zand L, Sethi S, Giesen C, Lieske JC, Fervenza FC. Noninvasive diagnosis of primary membranous nephropathy using phospholipase A2 receptor antibodies. Kidney international. 2019 Feb 1;95(2):429-38.
- Qin W, Beck LH, Zeng C, Chen Z, Li S, Zuo K, Salant DJ, Liu Z. Anti-phospholipase A2 receptor antibody in membranous nephropathy. Journal of the American Society of Nephrology. 2011 Jun 1;22(6):1137-43.

Excellent, thank you, Keep up the good work