MINI-REVIEW
Understanding APOL1-Mediated Kidney Disease: A Deep Dive into Epidemiology, Pathophysiology, and Therapeutic Opportunities

By Dr. Edward Kwakyi
Nephrologist
Specialist Physician and Senior Lecturer
Medicine and Therapeutics
University of Ghana Medical School
Introduction
APOL1-mediated kidney disease is a significant health concern, particularly among individuals of African descent. This condition is linked to genetic variations in the APOL1 gene, which can lead to various forms of kidney disease, including focal segmental glomerulosclerosis (FSGS) and hypertension-attributed chronic kidney disease (CKD). Here, we explore the epidemiology, pathophysiology, latest research, and emerging therapeutic opportunities for APOL1-mediated kidney disease.
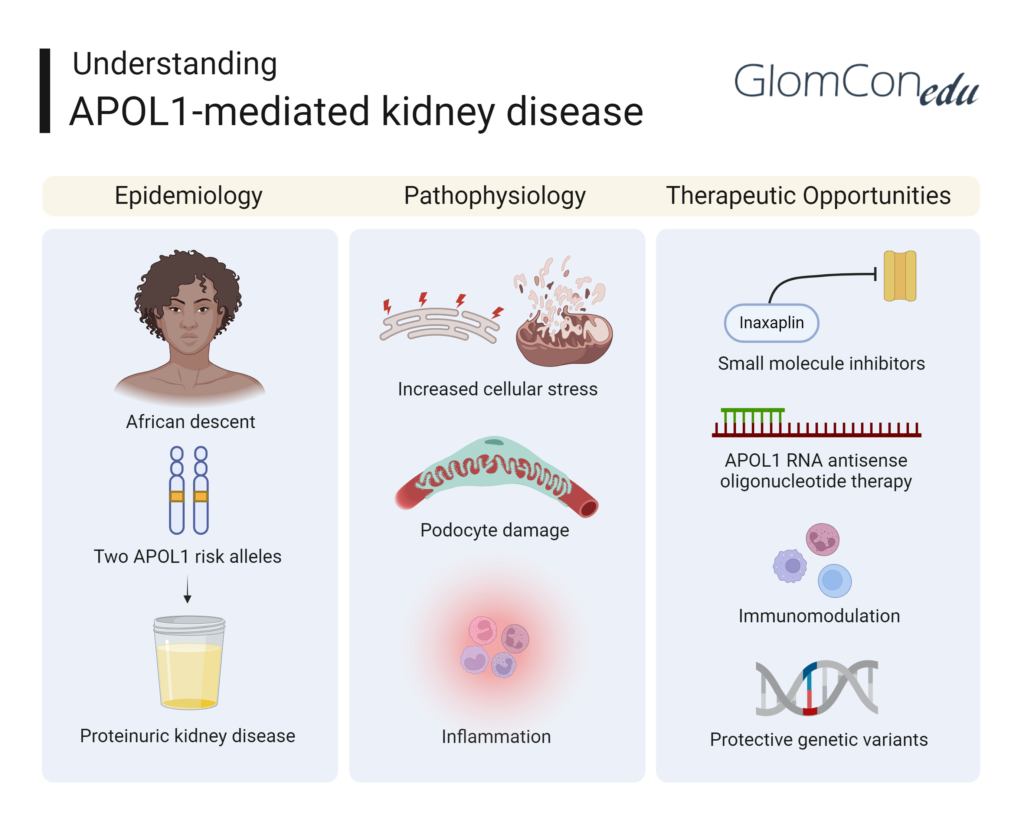
Epidemiology
APOL1-mediated kidney disease disproportionately affects people of African ancestry due to the higher prevalence of APOL1 risk variants (G1 and G2) in this population. These variants are rare or virtually absent in individuals of non-African descent. It is estimated that about 13% of African Americans carry two APOL1 risk alleles, putting them at a significantly higher risk for developing kidney disease compared to those without these variants. Global data suggests a much higher frequency of the high-risk APOL1 variants in West Africa (mainly Ghana and Nigeria), where approximately 25% of the population carries two APOL1 risk alleles. Other geographic regions on the African continent do not reflect a similarly high prevalence. Similar disparities have been found in Hispanic, Latino, and Afro-Caribbean communities.
The discovery of the APOL1 risk alleles has provided insight into the increased incidence of kidney disease in African American populations, who experience kidney failure at rates three to four times higher than white Americans. This disparity underscores the need for targeted research and tailored therapeutic approaches.
Pathophysiology
APOL1, or apolipoprotein L1, is a protein that plays a crucial role in innate immunity, particularly in protecting against Trypanosoma brucei, the parasite responsible for African sleeping sickness. The G1 and G2 variants of APOL1 evolved to provide resistance against this parasite, but they also predispose individuals to kidney disease.
The exact mechanisms by which APOL1 variants cause kidney damage are still being elucidated. However, research suggests that the toxic effects of the risk variants may be due to:
- Increased Cellular Stress: APOL1 variants can lead to endoplasmic reticulum stress and mitochondrial dysfunction, resulting in cell injury and death.
- Podocyte Damage: Podocytes, specialized cells in the kidney glomerulus essential for filtration, are particularly susceptible to APOL1-induced damage. This damage can lead to the development of FSGS and other glomerulopathies.
- Inflammation: APOL1 risk variants may promote inflammatory responses, further contributing to kidney injury and disease progression.
Breakthroughs in Unravelling the Pathogenesis of APOL1-Mediated Kidney Disease
Recent studies have advanced our understanding of APOL1-mediated kidney disease. Key findings include:
- Genetic Insights: The identification of additional genetic modifiers that influence the risk and severity of kidney disease in individuals with APOL1 variants.
- Biomarkers: Efforts to identify biomarkers that can predict disease progression and response to therapy.
- Pathogenic Pathways: Better characterization of the cellular and molecular pathways involved in APOL1-induced kidney injury, providing potential targets for therapy.
New Therapeutic Opportunities
The growing understanding of APOL1-mediated kidney disease has spurred the development of novel therapeutic strategies. Some promising approaches include:
- Small Molecule Inhibitors: Researchers are investigating small molecules that can inhibit the toxic effects of APOL1 variants. For example, Inaxaplin has shown promise in early clinical trials.
- APOL1 RNA antisense oligonucleotide therapy: Basic science studies have shown that antisense RNA can ameliorate experimentally induced proteinuria in mice.
- Immunomodulation: Therapies are being explored to modulate the immune response, reducing inflammation and protecting kidney function.
- Protective Genetic Variants: Identifying and harnessing protective genetic variants that counteract the effects of APOL1 risk alleles may provide a novel therapeutic avenue.
Conclusion
APOL1-mediated kidney disease represents a critical area of research with significant implications for public health, particularly in populations of African descent. Advances in our understanding of the disease’s epidemiology and pathophysiology and the development of targeted therapies offer hope for better management and potential cures in the future. Continued research and collaboration are essential to translating these discoveries into clinical practice, ultimately improving outcomes for those affected by this challenging condition.
For further readings, see also: https://www.glomcon.org/emerging-therapeutics/emerging-therapeutics-for-apol1-mediated-kidney-disease
References
- https://www.seminarsinnephrology.org/article/S0270-9295(13)00081-8/abstract
- https://www.akdh.org/article/S1548-5595(14)00100-1/abstract
- https://www.nejm.org/doi/full/10.1056/NEJMoa2202396
- https://www.science.org/doi/10.1126/science.1193032
- https://insight.jci.org/articles/view/126124
- https://journals.lww.com/cjasn/pages/articleviewer.aspx?year=2021&issue=02000&article=00020&type=Fulltext