DISEASE MANAGEMENT
Approach to Pregnancy in Lupus Nephritis
Disclaimer: This article does not provide medical advice. The information presented in this article is for educational purposes only. It is not intended to substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified healthcare provider if you have any questions regarding a medical condition or treatment.

By Dr. Benjamin Tan, MB, BCh, BaO, MRCP (UK)
Systemic lupus erythematosus (SLE) is a chronic, multi-system, autoimmune disorder affecting predominantly women, particularly those of childbearing age. Pre-pregnancy evaluation is vital for all SLE patients with childbearing potential to maximize the chance of a successful pregnancy and minimize risks to both mother and baby. Data from a systematic review and meta-analysis have shown that active lupus nephritis (LN) is associated with poor pregnancy outcomes.
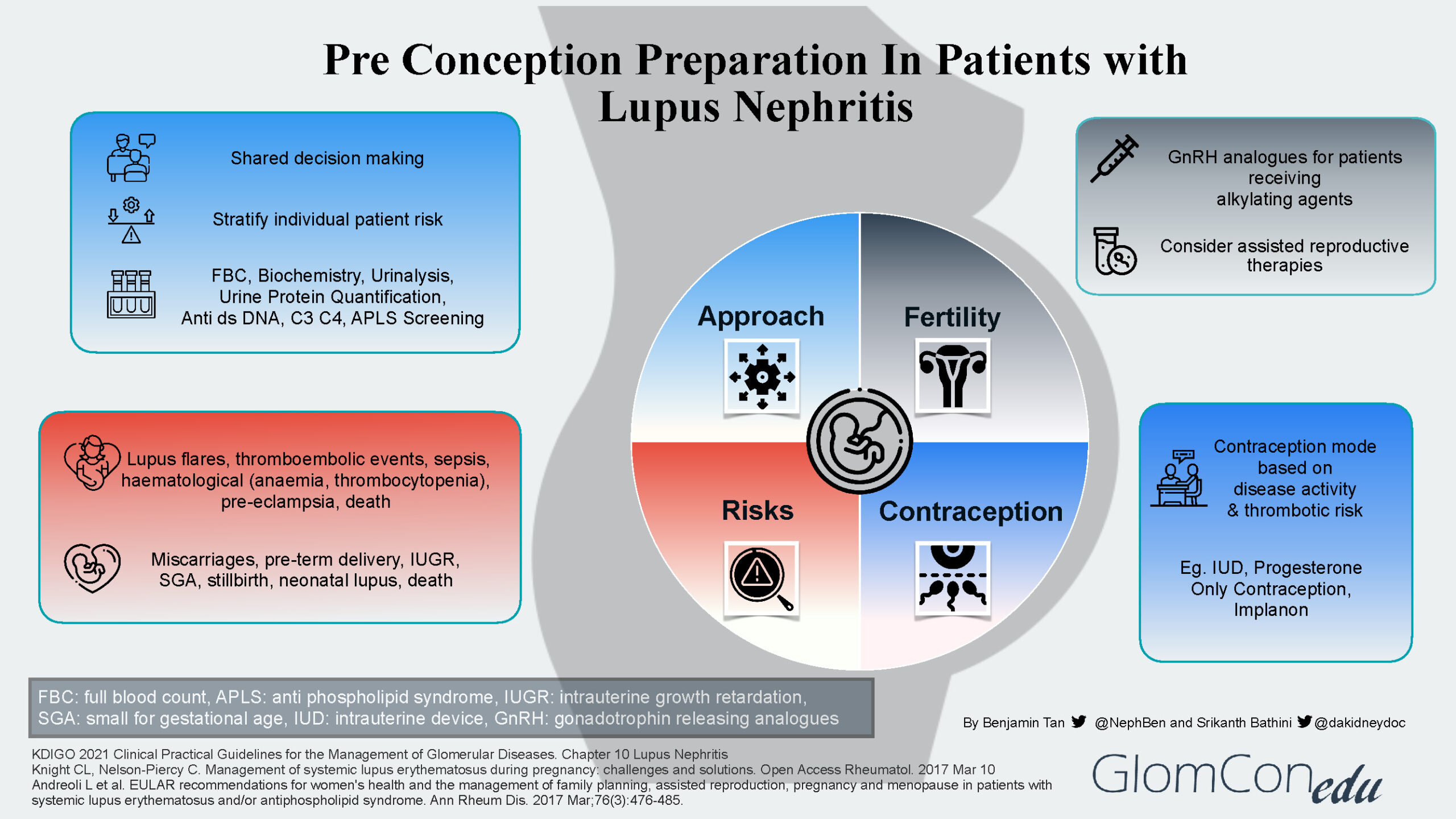
The infographic below summarizes the four main aspects to address during the pre-conception preparation of patients with LN.
Approach
Pre-pregnancy evaluation is imperative for all women with LN to stratify them into different risk profile groups. A multidisciplinary team would be valuable in determining the best approach to their care.
Generally, women of childbearing age with LN can be divided into 3 groups, namely:
- Inactive/quiescent LN*
- Active LN disease*
- LN with severe impairment of organ function and/or pre-existing severe organ damage.
*An assessment of disease activity is made using clinical, laboratory (biochemistry, urine analysis, quantification, serologies), and histological parameters (renal biopsy)
Risks
Pregnancy may cause LN flares with progressive kidney disease. In addition, SLE itself may cause a variety of obstetric-related complications, notably pre-eclampsia and thromboembolic events, as well as neonatal-related complications such as pre-term delivery and miscarriages.
While explaining these risks, it is important to remember that with close multidisciplinary surveillance, patients in complete remission or partial remission with low disease activity can have favorable pregnancy outcomes.
Fertility
Given the inherent risks of infertility (menstrual irregularities and premature ovarian failure) associated with cyclophosphamide, a mycophenolic acid analog (MPAA) based regimen is the preferred initial therapy of proliferative LN. It is also strongly recommended that all women of childbearing age with SLE be counseled about fertility issues, especially the adverse outcomes associated with increasing age and the use of alkylating agents. If the patient is to receive an alkylating agent, then fertility preservation methods such as gonadotropin releasing hormone analogs (GnRH) should be considered. Assisted reproductive therapies (ART) such as in vitro fertilization protocols and ovulation induction treatments can also be considered in LN patients with stable or inactive disease.
Contraception
To prevent unwanted pregnancies, especially during active disease and those receiving teratogenic medications, women with LN should be advised regarding contraception methods. The mode of contraception is decided based on disease activity, thrombotic risk, and patient preference. Many options are available such as the intrauterine device (IUD) and progesterone-only contraception as pills, intramuscular depot injections, or subdermal implants.
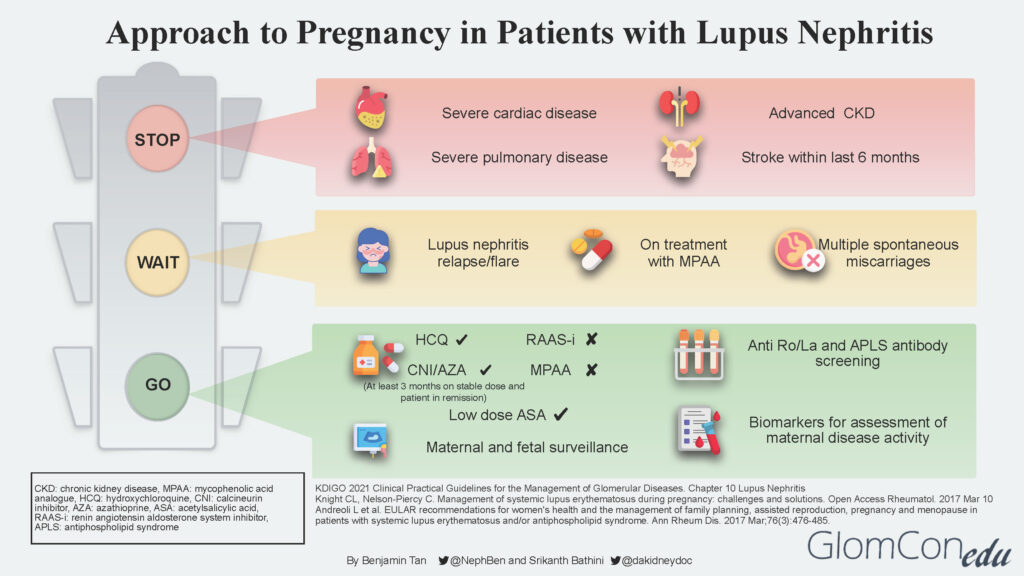
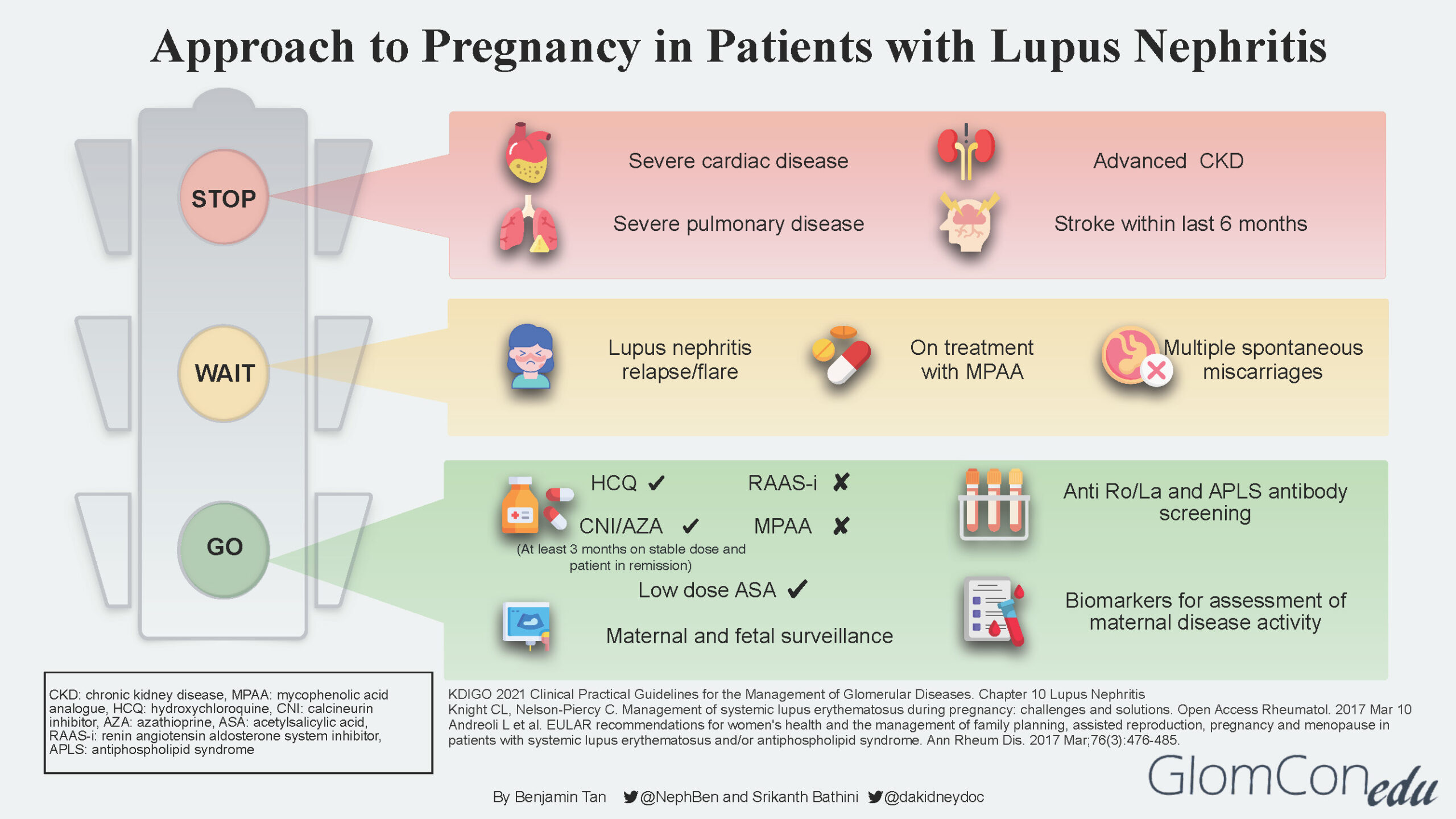
The infographic below depicts the suggested approach and direction for each group of patients after a comprehensive risk assessment is done.
STOP
LN patients with concurrent severe organ impairment or damage (Group 3) should be counseled regarding the risks for disease progression that could lead to end-organ failure and pregnancy-related risks to both mother and baby. Women in this group should be discouraged from pursuing pregnancy. Instead, they should be encouraged to consider alternative options such as adoption and surrogacy. The table below describes the various conditions in detail.
| Conditions | Comment |
|---|---|
| Severe cardiac disease | Moderate to severe heart failure, severe valvulopathy with evidence of pulmonary hypertension |
| Severe pulmonary disease | Severe restrictive lung disease with pulmonary hypertension |
| Advanced chronic kidney disease (CKD) | CKD stages 4-5 |
| Stroke | Stroke occurrence within the last 6 months |
WAIT
Patients with active LN disease should be advised to avoid pregnancy for at least 6 months after LN is inactive. LN at conception confers a higher risk of flare during pregnancy, even in women in remission. Studies suggest that the risk of a lupus flare either during or immediately after pregnancy among patients with pre-existing lupus nephritis is between 30–60%.
Delaying pregnancy plans would ensure patients are well optimized for successful pregnancy outcomes in the future. General pre-conception advice is also given, including a discussion of appropriate contraception methods.
GO
Patients with inactive LN disease (Group 1) are informed that this is the safest (lowest risk) window/period to proceed with pregnancy planning. Before embarking on pregnancy, screening for antiphospholipid syndrome (APS) antibodies is strongly recommended for all SLE patients. Disease activity should be continuously monitored throughout pregnancy using available lupus biomarkers such as urinalysis, urine protein quantification, complement levels as well as anti-dsDNA levels.
It is imperative to discontinue teratogenic medication such as MPAA and transition to non-teratogenic therapy such as calcineurin inhibitor (CNI) or azathioprine. A “wash out” period of at least 3 months is advised for such patients. Renin-angiotensin-aldosterone system (RAAS) blockade agents should also be discontinued. If antihypertensive therapy is required, pregnancy-safe medications such as labetalol, nifedipine, and methyldopa should be considered.
The use of hydroxychloroquine (HCQ) is generally safe throughout pregnancy, although a recent study showed a small increase in the risk of congenital malformations among HCQ-exposed pregnancies. The significant benefit of HCQ in reducing the risk of flares during and after pregnancy needs to be weighed against the small increased risk of congenital malformations. The increased risk of flares has also been associated with the discontinuation/taper of HCQ.
Low-dose aspirin (<100mg) and calcium supplements are recommended before 16 weeks of gestation as prophylactic therapy against pre-eclampsia.
Throughout the antepartum period, patients will undergo regular maternal and fetal surveillance by a multidisciplinary team consisting of the nephrologist, obstetrician, and rheumatologist.
Acknowledgments
Infographic icons made by Smashicons, Vitaly Gorbachev, Freepik, Surang, Flat Icons, Amethyst Design, Juicy_fish, noomtah, Eucalyp from www.flaticon.com

This is a good aproach of this defases .