TREATMENT
Cyclophosphamide In Rapidly Progressive IgA Nephropathy
The authors have no conflicts of interest to declare.
GlomCon Editors with significant contribution to the development of this article include Haresh Selvaskandan, Paolo Nikolai So, and Nasim Wiegley.
Abstract
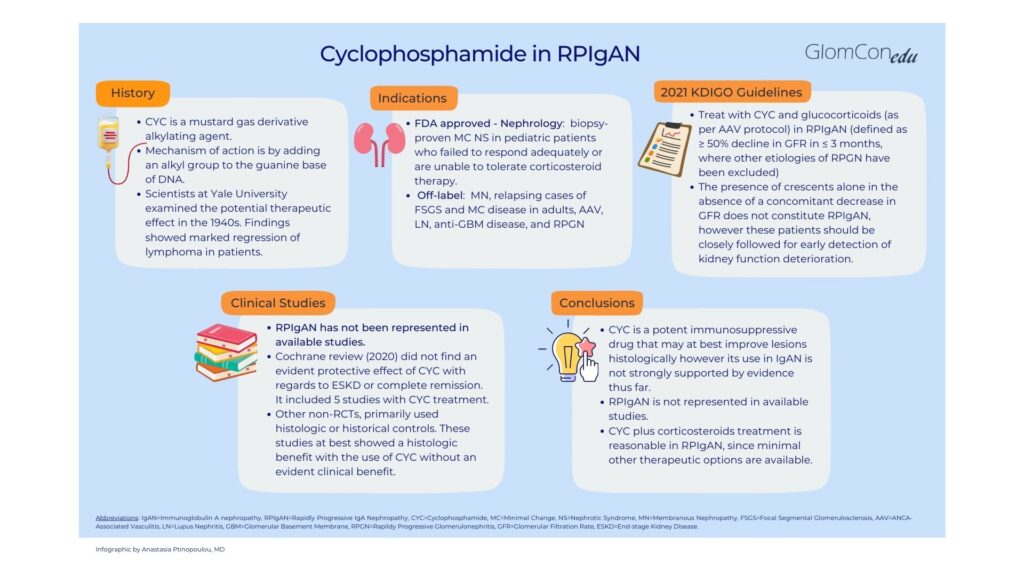
Cyclophosphamide is an alkylating agent that acts by adding an alkyl group to the guanine base of DNA. It is a potent immunosuppressive used to treat many glomerular diseases. However, in primary IgA nephropathy (IgAN), a disease with great variability in histologic and clinical manifestations, available studies lack homogeneity and do not provide significant evidence to support cyclophosphamide use. The KDIGO 2021 Guideline for the Management of Glomerular Disease recommends cyclophosphamide and glucocorticoids only in rapidly progressive IgAN (RPIgAN), emphasizing that the presence of crescents alone does not constitute RPIgAN. This article aims to provide an overview of the literature which evaluates cyclophosphamide in IgAN.
Introduction/ History – Origins of the drug
Cyclophosphamide is an alkylating agent used as a cytotoxic chemotherapy drug. Its origin dates back to the 1940s when scientists at Yale University examined the potential therapeutic effect of mustard gas derivatives (used during World War I as a chemical weapon). The observation that people exposed to mustard gas had marked depletion of bone marrow and lymph nodes initiated research on the effect of mustard compounds; findings showed marked regression in lymphoma patients and led to the synthesis of alkylating compounds, including cyclophosphamide. (1)
Mechanism of action
Alkylating drugs act by adding an alkyl group to the guanine base of DNA. This leads to cross-linking of DNA and prevents DNA strands from being separated for transcription or causes mutations and DNA fragmentation. Protein synthesis is impaired, resulting in apoptosis of rapidly proliferating malignant cells. (2)
Indications in glomerular diseases – FDA-approved and off-label use
Cyclophosphamide is FDA-approved for biopsy-proven minimal change nephrotic syndrome in pediatric patients who failed to respond adequately or are unable to tolerate corticosteroid therapy. (3) Off-label uses for glomerular diseases are numerous and include membranous nephropathy, relapsing cases of FSGS and minimal change disease in adults, ANCA-associated vasculitis, lupus nephritis, anti-GBM disease, and rapidly progressive glomerulonephritis. (4)
Cyclophosphamide in IgAN
The 2021 KDIGO Guideline for the Management of Glomerular Disease recommends that patients with rapidly progressive primary IgAN (RPIgAN), defined as a ≥ 50% decline in GFR in ≤ 3 months, be offered treatment with cyclophosphamide and glucocorticoids as per ANCA-associated vasculitis protocol. (5). Available data regarding cyclophosphamide use in IgAN is poor but virtually non-existent for RPIgAN. Of note, the KDIGO 2021 guidelines emphasize that the presence of crescents alone in the absence of a concomitant decrease in GFR does not constitute RPIgAN. (5)
Clinical trials
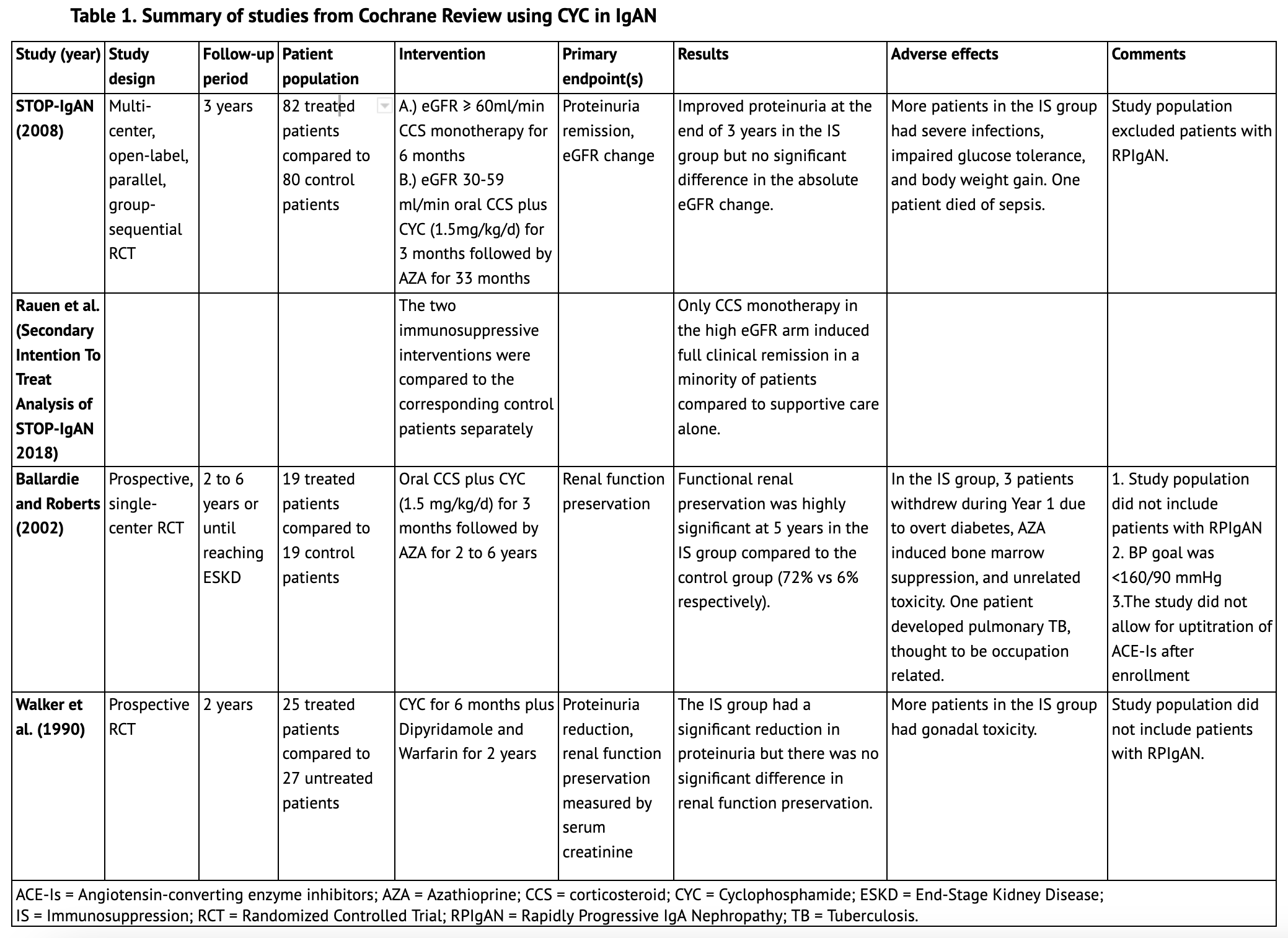
The 2020 Cochrane Review update regarding immunosuppression in IgAN includes studies up to September 2019. (6) Five studies included cyclophosphamide in the immunosuppressive treatment regimen; three of these are summarized in Table 1. The largest one, STOP-IgAN (2008), included 82 patients in the immunosuppression arm with three years of follow-up. Patients who had an eGFR of at least 60 ml/min received glucocorticoid monotherapy for six months, and patients with an eGFR between 30 ml/min and 59 ml/min received oral prednisolone plus cyclophosphamide (1.5 mg/kg/d) for three months followed by azathioprine for the subsequent 33 months. Notably, patients with a rapid decrease in eGFR (defined as a > 30% decrease from the start of the run-in phase) were not randomized. This pooled analysis showed that immunosuppression was superior to supportive care alone in attaining full clinical remission at the end of the three-year study period primarily due to proteinuria remission; however, there was no significant difference in the absolute eGFR change at 36 months between the two groups. In a secondary analysis, where the two immunosuppressive arms were compared to the corresponding control patients separately, only corticosteroid monotherapy in the high eGFR arm induced significant full clinical remission in a minority of patients compared to supportive care alone. Patients with RPIgAN, however, were completely excluded from this study. (7)
Another single-center study included in the Cochrane Review, from Ballardie and Roberts (2002), followed 38 IgAN patients whose serum creatinine levels were greater than 130 μmol/L and rising by at least 15% in the year before entry. They were randomized to receive either oral prednisolone plus cyclophosphamide (1.5 mg/kg/d) for three months, followed by azathioprine for a minimum of 2 years or to a control group that received standard supportive treatment. Functional renal preservation was highly significant at five years in the immunosuppressive group compared to the control group (72% v 6%, respectively). The study, however, elected for a higher BP goal (16090mmHg) after trial entry compared to current recommendations and also did not allow for up-titration of angiotensin-converting enzyme inhibitors (ACE-Is) after enrollment, a key tool to optimizing supportive care. Moreover, the study population also did not include patients with RPIgAN. (8)
Another study by Walker et al. (1990) randomized 52 patients to either cyclophosphamide for six months plus dipyridamole and warfarin for two years or to supportive care. At the time of entry, participants in the immunosuppressive arm did not have significant differences in mean serum creatinine or urine protein excretion compared to patients who did not receive immunosuppression. Although the cyclophosphamide group significantly reduced proteinuria compared to the control group at the end of the trial, there was no significant difference in renal function preservation between the two arms. This study also did not include patients with RPIgAN. (9)
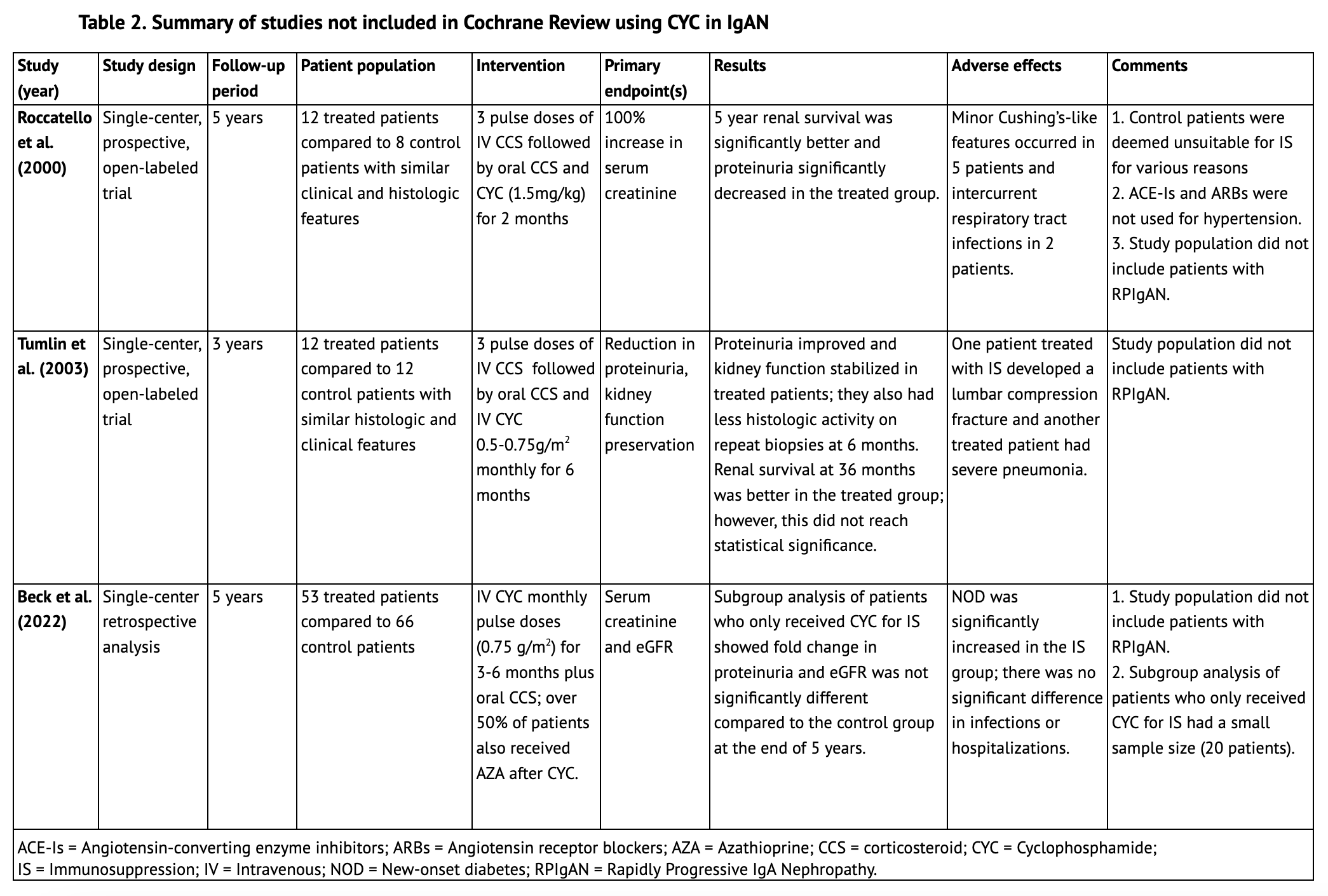
The Cochrane Review did not find an evident protective effect of cyclophosphamide in IgAN with regards to renal function preservation; however, patients with RPIgAN as defined by current KDIGO criteria were not represented in the aforementioned studies. (6) Other lower quality, non-randomized controlled trials, not included in the Cochrane review, primarily used histologic or historical controls. These studies, summarized in Table 2, did not include patients with RPIgAN either and at best showed a histologic benefit with the use of cyclophosphamide without an evident clinical benefit.
Roccatello et al. (2000) treated twelve IgAN patients with florid glomerular changes (8-60% cellular crescents) with a two month course of cyclophosphamide (1.5mg/kg) in addition to corticosteroids. These were compared to a control group of eight patients with similar histologic findings who were deemed unsuitable for immunosuppression for various reasons; therefore they may not represent matching groups. They found significantly better renal survival at the end of 5 years in the treatment group, as well as significantly decreased proteinuria. However, ACE-Is or angiotensin receptor blockers (ARBs) were not used for hypertension treatment. Moreover, patients with RPIgAN were not included in the study. (10)
Tumlin et al. (2003) conducted a prospective open-label trial where they treated a group of twelve IgAN patients with at least 10% cellular crescents with three methylprednisolone pulse doses, followed by oral prednisolone and intravenous cyclophosphamide 0.5-0.75g/m2 monthly for 6 months. These patients were compared to 12 historical control patients who were untreated and had similar degrees of histologic activity. Although renal survival was better at 36 months for the treatment group (8.3% of patients in the treated group compared to 41.7% of patients in the historical controls reached ESKD), this value did not reach statistical significance. Similar to the prior studies, this study also did not include patients with RPIgAN. (11)
In another retrospective analysis that examined IgAN patients from a single center over a period of twenty years, Beck et al. (2022) compared fifty-three patients with IgAN who received monthly pulse doses of cyclophosphamide for 3-6 months and glucocorticoids with over half also receiving azathioprine to a control group of sixty-six patients who received supportive care. Subgroup analysis of patients who received cyclophosphamide alone showed a significantly improved eGFR fold change and proteinuria reduction during the course of the study however this was not statistically different at the end of the 5 year period. Patients with RPIgAN were not included in this study either. (12)
Conclusion
Cyclophosphamide is a potent immunosuppressive drug that may at best improve lesions histologically however its use in IgAN is not strongly supported by evidence thus far. Patients with RPIgAN, as defined by current KDIGO criteria, have been excluded from available studies; therefore evidence to support its usage in this patient population is minimal. Although some lower quality evidence suggests histological benefit with combined cyclophosphamide and corticosteroid therapy in patients with active inflammatory lesions, high quality RCTs are needed in order to determine clinical benefit. KDIGO guidelines recommend the use of cyclophosphamide and glucocorticoids only for RPIgAN, based on data derived from other glomerular diseases and the lack of other therapeutic options available to rescue this high risk cohort from end stage kidney disease.
References
- DeVita VT, Chu E. A History of Cancer Chemotherapy. Cancer Res. 2008 Nov 1;68(21): 8643–8653. https://doi.org/10.1158/0008-5472.CAN-07-6611
- Ralhan R, Kaur J. Alkylating agents and cancer therapy. Expert Opin Ther Patents. 2007;17(9):1061-1075. https://doi.org/10.1517/13543776.17.9.1061
- FDA Drug Label for Cyclophosphamide. Available from: https://nctr-crs.fda.gov/fdalabel/ui/spl-summaries/criteria/393772
- Ponticelli C, Escoli R, Moroni G. Does cyclophosphamide still play a role in glomerular diseases? Autoimmun Rev. 2018 Oct;17(10):1022-1027. https://doi.org/10.1016/j.autrev.2018.04.007
- KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021 Oct;100(4S):S1–S276. https://doi.org/10.1016/j.kint.2021.05.021
- Natale P, Palmer SC, Ruospo M, et al. Immunosuppressive agents for treating IgA nephropathy (Review). Cochrane Database of Systematic Reviews. 2020:3;CD003965. https://doi.org/10.1002/14651858.CD003965.pub3
- Rauen T, Fitzner C, Eitner F, et al. Effects of Two Immunosuppressive Treatment Protocols for IgA Nephropathy. J Am Soc Nephrol. 2018 Jan;29(1):317-325. https://doi.org/10.1681/ASN.2017060713
- Ballardie FW, Roberts ISD. Controlled Prospective Trial of Prednisolone and Cytotoxics in Progressive IgA Nephropathy. J Am Soc Nephrol 2002;13:142–148. https://doi.org/10.1681/asn.v131142
- Walker RG, Yu SH, Owen JE, et al. The treatment of mesangial IgA nephropathy with cyclophosphamide, dipyridamole and warfarin: a two-year prospective trial. Clin Nephrol. 1990 Sep;34(3):103-7. https://pubmed.ncbi.nlm.nih.gov/2225560/
- Roccatello D, Ferro M, Cesano G, et al. Steroid and cyclophosphamide in IgA nephropathy. Nephrol Dial Transplant. 2000 Jun;15(6):833-5. https://doi.org/10.1093/ndt/15.6.833
- Tumlin JA, Lohavichan V, Hennigar R. Crescentic, proliferative IgA nephropathy: clinical and histological response to methylprednisolone and intravenous cyclophosphamide. Nephrol Dial Transplant. 2003 Jul;18(7):1321–1329. https://doi.org/10.1093/ndt/gfg081
- Beck N, Walz G, Schneider J. Effect of Cyclophosphamide and Glucocorticoid Therapy in IgA Nephropathy: A Single-Center Retrospective Analysis. Kidney360. 2022 Jan 19;3(3):506-515. https://doi.org/10.34067/KID.0006702021