CASE REPORT
De Novo Glomerulonephritis with Chronic Antibody Mediated Rejection in a Kidney Transplant Recipient
Srilekha Sridhara M.D1*, Tala Saleh M.D2, Ahmed-Jordan Salahat M.D2, Mohammed Sikder M.D3, Abd Assalam Qannus M.D1, Bekir Tanriover M.D, MBA1, Samih H. Nasr M.D4, Aneesha Shetty M.D1, Venkatesh Ariyamuthu M.D MBA1
Authors’ Affiliations:
1 University of Arizona, Tucson, AZ
2 University of Jordan, Amman, Jordan
3 Southwest Kidney Institute, Tucson, AZ
4 Mayo Clinic, Rochester, MN
* Corresponding Author
Authors’ Full Names and Academic Degrees:
Srilekha Sridhara M.D, Nephrology fellow 1
Tala Saleh M.D, Intern 2
Ahmed-Jordan Salahat M.D, Intern 2
Mohammed Sikder M.D, Nephrology Specialist 3
Abd Assalam Qannus M.D 1
Bekir Tanriover M.D, MBA, Chairman, Division of Nephrology 1
Samih Nasr M.D, Professor of Laboratory Medicine and Pathology 4
Aneesha Shetty M.D, Director of Living Donor Kidney Transplantation 1
Venkatesh Ariyamuthu M.D, MBA Director of Transplantation 1
Address for Correspondence:
Srilekha Sridhara M.D
Nephrology fellow
1501 N Campbell Ave. Tucson, AZ 85724
Srilekha.sridhara@bannerhealth.com
Case Presentation
A 61-year-old male with end-stage kidney disease due to congenital solitary kidney, received a deceased donor kidney transplantation in 1995. Post-transplant, baseline creatinine was 1.6 to 1.8 mg/dL and he was maintained on an immunosuppressive regimen of azathioprine, cyclosporine, and prednisone. In 2022, he developed lower extremity edema and foamy urine for a few weeks. Labs revealed blood urea nitrogen of 44 mg/dL, creatinine 3.03 mg/dL with eGFR of 23 mL/min per 1.73 m², albumin 4.1 g/dL, total protein 7.3 g/dL, urine microalbumin to creatinine ratio was 262 mg/g and urine protein to creatinine ratio was 447 mg/g. Kidney ultrasound showed mild cortical thinning. Serum complements and free light chain ratio were normal. ANA antibody, donor specific antibodies, serum and urine monoclonal proteins were undetectable. Age-appropriate cancer screening was completed including PSA testing which was normal at 0.89 ng/mL and colonoscopy did not reveal any malignancy. CMV PCR, BK PCR were undetectable. HIV 1/2 antibody, EBV VC IgM, hepatitis B surface antigen and hepatitis C antibody were non-reactive.
Kidney transplant biopsy revealed chronic antibody mediated rejection (with transplant glomerulopathy, glomerulitis, and peritubular capillaritis, C4d-negative). Immunofluorescence revealed 6 glomeruli with 1-2+ smudgy mesangial and segmental glomerular capillary wall positivity for IgG with 1+ kappa, 1+ lambda, 1+ IgG2, trace C3, trace IgG1, and negative IgG3, IgG4 and C1q staining. Electron microscopy revealed segmental mesangial deposition of randomly oriented straight fibrils, and special staining was performed. (Figure 1).
- What is the differential for the glomerular fibrils?
- What investigative studies should be undertaken?
- How should the patient be managed?
- What are the expected outcomes?
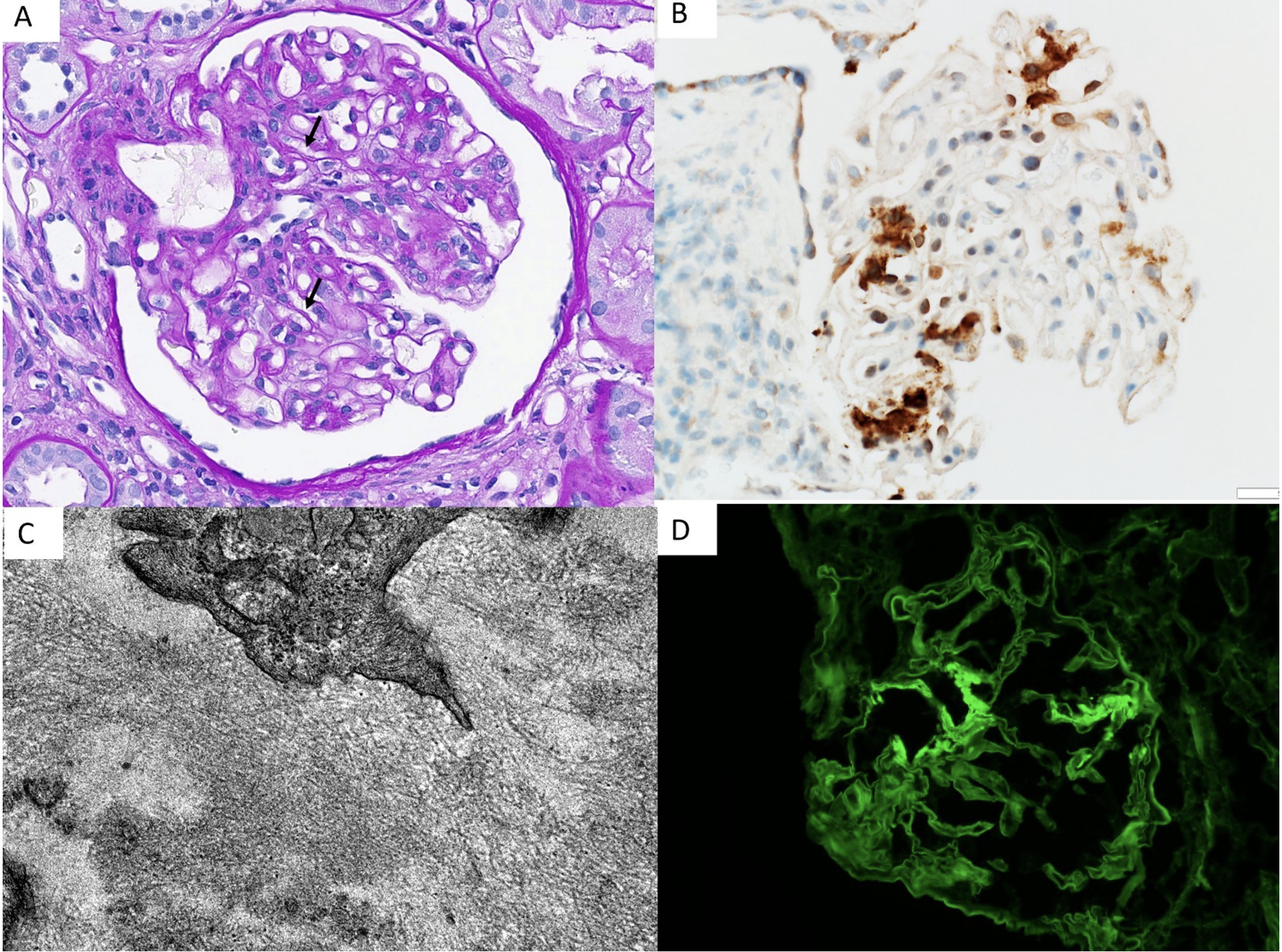
Figure 1. Figures A-D showing segmental duplication of the glomerular basement membrane (arrows), intracapillary marginating mononuclear cells, and mild mesangial expansion. (A, PAS stain X400), segmental smudgy mesangial DNAJB9 staining (B), mesangial randomly arranged fibrils on electron microscopy (C, X50,000) and smudgy segmental mesangial staining for IgG on Immunofluorescence (D).
Discussion
Determination of a de novo post-transplant glomerular disease can be challenging because of confounding role of rejection, immunosuppression (IS), hypertension and infection. In a study of Canadian population by Chailimpamontree et al., de novo focal segmental glomerulosclerosis was the most common glomerular disease (42%) (1). The figure 1 in this study shows that the rate of de novo post-transplant glomerulonephritis was 3.5% as noted in 26 out of 734 originally biopsied cases. Fibrillary glomerulonephritis (FGN) is reported in 0.8-1.5% of native kidney biopsies and de novo occurrence of post-transplant FGN is very rare (2).
- What is the differential for glomerular fibrils?
The differential diagnosis for glomerulopathies with organized deposits is based on the fibril characteristics as shown in Table 1. FGN is a proliferative glomerulopathy characterized by randomly arranged fibrils deposited in mesangium, glomerular basement membrane or both (3).
Immunofluorescence (IF) in FGN demonstrates polyclonal IgG, complement and DNAJB9 positive that are mostly Congo red negative. DNAJB9, a chaperone from the DNAJ family, is upregulated under specific stress conditions and protects against cell death (4). In normal kidneys, DNAJB 9 is present in low levels in podocytes, mesangial and endothelial cells and FGN is the only disease associated with large amounts of extracellular deposition. DNAJB9 staining is found to be highly sensitive and specific for diagnosing FGN (5). In our patient, DNAJB9 immunohistochemical staining was positive in certain glomerular mesangial areas.
Table 1. Differential diagnosis for glomerulopathies with organized deposits based on fibril characteristics.
- What investigative studies should be undertaken to determine etiology for FGN?
FGN can be associated with multiple diseases as shown in Table 2. Screening for these conditions at diagnosis and on follow up is recommended. Our patient underwent multiple diagnostic investigations, and the results were all non-contributory.
Table 2. Underlying diseases associated with FGN (7)
- How should FGN patients be managed?
There are no randomized control trials that guide management decisions. Supportive care with dietary sodium restriction, blood pressure control, antiproteinuric and lipid-lowering therapies are recommended for all patients. IS with Rituximab (RTX) may also be considered after assessing the risks and benefits. Depending on the response (complete vs. partial) and CD 19 B-cell count, RTX redosing could be considered (4). Our patient received two doses of Rituximab 1gm IV 2 weeks apart. His immunosuppression was modified, and he was placed on Mycophenolate mofetil 500 mg twice daily, Tacrolimus 1 mg twice daily and prednisone taper. He did not receive any further doses of Rituximab.
- What are the expected outcomes for FGN?
The prognosis for FGN is generally poor and nearly half of the patients progress to renal failure within few years of diagnosis (3). Post-transplant FGN histologic recurrence however has an indolent course. In a case series of 14 FGN patients who underwent renal transplantation, 3 patients experienced FGN recurrence with a notably longer follow-up duration (14.2 years vs. 4.4 years) compared to those without recurrent FGN (6). Of these 3 patients, 1 patient lost the allograft in the setting of chronic antibody-mediated rejection. Our patient likely had a milder presentation without sclerosis, he responded to RTX and experienced decreased edema, and the creatinine is now 2.2 mg/dL and urine protein to creatinine ratio is at 132 mg/g. We will continue monitoring his symptoms, serum creatinine, and proteinuria every 3 months.
Final Diagnosis
De novo fibrillary GN with chronic antibody mediated rejection in a renal transplant recipient.
Support: None.
Financial Disclosure: The authors declare that they have no relevant financial interests.
Patient Protections: The authors declare that they have obtained consent from the patient reported in this article for publication of the information about him that appears within this article.
References
- Chailimpamontree W, Keown PA; Genome Canada Biomarkers in Transplantation Group. Probability, predictors, and prognosis of posttransplantation glomerulonephritis. J Am Soc Nephrol. 2009 Apr;20(4):843-51. doi: 10.1681/ASN.2008050454.
- Karanam K, Decker B. Fibrillary Glomerulonephritis. Nephrol Rev. 2012;4. doi:10.4081/nr.2012.e16
- Nasr SH, Valeri AM, Cornell LD, et al. Fibrillary glomerulonephritis: a report of 66 cases from a single institution. Clin J Am Soc Nephrol CJASN. 2011;6(4):775-784. doi:10.2215/CJN.08300910
- Andeen NK, Yang HY, Dai DF, MacCoss MJ, Smith KD. DnaJ Homolog Subfamily B Member 9 Is a Putative Autoantigen in Fibrillary GN. J Am Soc Nephrol JASN. 2018;29(1):231-239. doi:10.1681/ASN.2017050566
- Nasr SH, Vrana JA, Dasari S, et al. DNAJB9 Is a Specific Immunohistochemical Marker for Fibrillary Glomerulonephritis. Kidney Int Rep. 2017;3(1):56-64. doi:10.1016/j.ekir.2017.07.017
- El Ters M, Bobart SA, Cornell LD, et al. Recurrence of DNAJB9-Positive Fibrillary Glomerulonephritis After Kidney Transplantation: A Case Series. Am J Kidney Dis Off J Natl Kidney Found. 2020;76(4):500-510. doi:10.1053/j.ajkd.2020.01.018
- Jordan L. Rosenstock, Glen S. Markowitz, Fibrillary Glomerulonephritis: An Update, Kidney International Reports, Volume 4, Issue 7, 2019, Pages 917-922, ISSN 2468-0249, https://doi.org/10.1016/j.ekir.2019.04.013.
