DIAGNOSTIC WORKUP
Basic Principles in Evaluating Glomerular Disease, The House of Deposits
Acknowledgment: Based on GlomCon’s Glomerular Disease Virtual Fellowship seminar “The Guiding Principles for Evaluation of Glomerular Disorders” by Dr. Richard Glassock.
Following an exciting first seminar, the GlomCon fellows learned about the principles governing glomerular disease evaluation. The House of Deposits dived into the literature to find answers to three pertinent related questions:
- What is the burden of glomerular disorders driven by systemic or secondary causes?
- How useful are the tools we have at our disposal for detecting and evaluating glomerular diseases?
- The aim of the game is to preserve kidney function – but do we measure kidney function the right way?
This report takes a closer look at these questions and examines the evidence for common practices.
Following an exciting first seminar, the GlomCon fellows learned about the principles governing glomerular disease evaluation. The House of Deposits dived into the literature to find answers to three pertinent related questions:

By Dr. Haresh Selvaskandan
Nephrology Fellow/KRUK Fellow
University Hospitals of Leicester NHS Trust
UK
Section 1: Excluding the secondaries – the first step in getting a GN diagnosis, right?
This report will highlight that identifying secondary drivers of glomerulonephritis (GN) is a critical first step in ensuring proper management is brought to the right patient. How important are secondary drivers of GNs? To answer this, let us start with a broader question:
How much “glomerular disease” is out there?
Calculating the true incidence or prevalence of GN is challenging. Although some present with clear clinical features (e.g., the nephrotic syndrome), others can remain wholly asymptomatic and evade detection; IgA nephropathy may only cause microscopic haematuria, and in the absence of any significant risk factors for progression (JAMA Intern Med. 2019;179:942), a patient who never gets tested may never get picked up.
Other GNs are slow burners – only presenting once the symptoms of chronic kidney disease (CKD) sets in. These patients may have atrophic kidneys and are, therefore, unlikely to receive a biopsy and a formal diagnosis. In the absence of clearly suggestive features, these patients are likely to be labeled CKD of unknown etiology or controversially, as hypertensive nephropathy, if they present with significant hypertension.
These features, in addition to the varying prevalence of GNs across the world (IgA nephropathy is so common in East Asia that there are school screening programs to detect it) and variable practice when it comes to undertaking a renal biopsy, it becomes clear why estimating the burden of GN can be challenging.
However, there is data to orientate us.
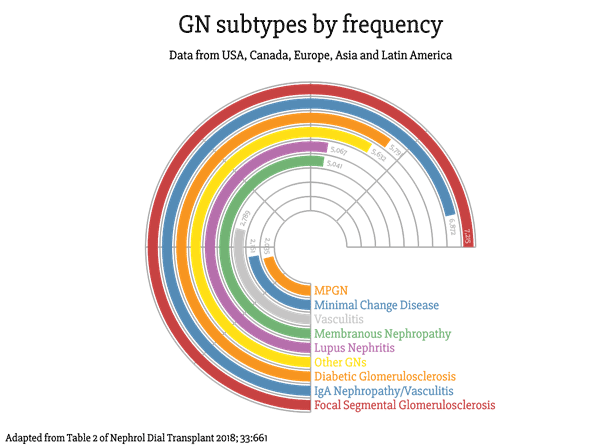
Renal registry data from the USA, UK, Australia, and New Zealand highlight GN as the third most common cause of end-stage kidney disease (ESKD). This number likely to be higher due to the arguments given above.
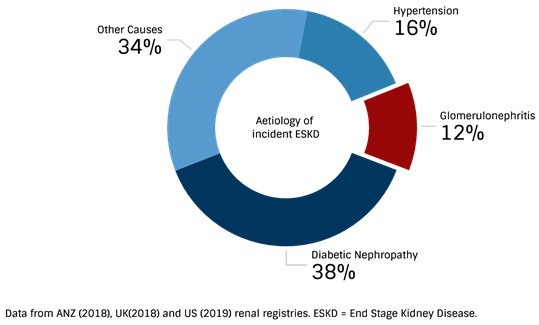
It is clear that in the world of nephrology, GNs certainly aren’t as rare as they may seem, accounting for at least one in 10 cases of every patient reaching ESKD.
GN’s are common – but do systemically driven or secondary GNs account for much?
The answer is almost definitely a yes. However, again the data is a little tricky to navigate.
Let’s look at the commonest reported causes of biopsy-proven GN (caveats discussed in question one apply).

Already on the list are three GNs that are inherently driven by systemic disease processes (Vasculitis, Lupus Nephritis, and Diabetic Glomeruloscerlosis). This leaves five GNs (we’ll get to ‘other GNs’ in a minute) that could, in theory, be primary.
Here’s where things become tricky.
Estimating the incidence or prevalence of secondary GNs is challenging for all the reasons described above and more. Records rarely list a GN as primary or secondary, so a retrospective analysis is usually not feasible. Furthermore, a GN will only be diagnosed as secondary if a cause is sought for it in the first place, which may not always be done. Nevertheless, below is a list of the remaining five GN’s from the above figure and the proportions that are likely to be driven by secondary causes. References are given for you to judge the validity of the assumptions made in the below figure, but reassuringly, it seems to match what we see anecdotally in practice.
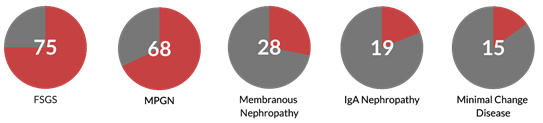
The proportion of GN accounted for by secondary drivers.
FSGS (Mayo Clin Proc 2017; 92:1772), MPGN (Hippokratia 2015; 19:314), Membranous Nephropathy (J Clin Pathol 1986; 39:1193), IgA Nephropathy (PLoS One 2019; 14:e0221014), Minimal Change Disease (Comprehensive Clinical Nephrology).
The ‘other GNs’ category is made up of less common GNs, most likely driven by secondary causes; the treemap below shows you what this category is made up of:
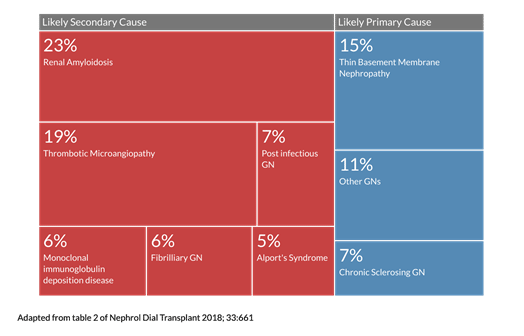
We can see that secondary or systemic processes play a sizable role in the generation of GNs. If we assume the above data is generalizable and merge it all, we end up with the following numbers:
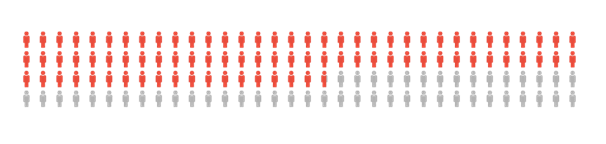
63.8% of GNs reported (based on the above information) could be driven by a systemic or secondary cause.
What drives secondary GNs?
Knowing that secondary GNs account for a large proportion of what we may see clinically, the next step to consider is what the drivers of secondary GNs are, and there are many. Knowing each of these causes is challenging, and recalling each of these causes in clinical practice is even more so.
A more practical approach would be to consider that most secondary/systemic causes of GNs generally fall into four categories:
Autoimmune

Malignant

Infectious

Drugs
It’s worth having a system to help tease out if one of these big players are likely in the mix.
Although no system will be a substitute for experience, one may help pick up an underlying cause that may otherwise be missed. Once a diagnosis of a GN is made histologically, then a focussed hunt for causes specific to that GN can be initiated (see table below), but for now, here are some systems that may be useful:
Rheumatological or autoimmune causes:
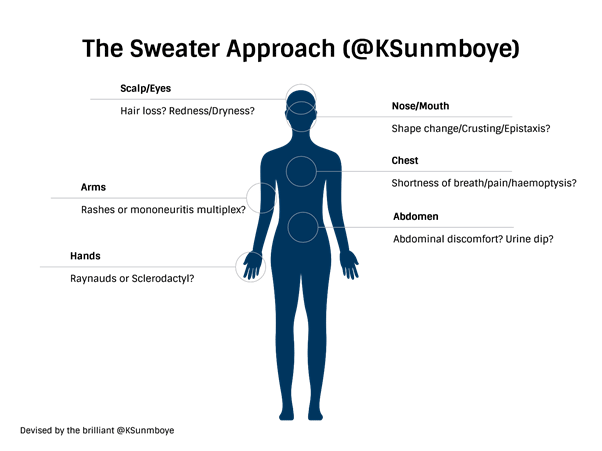
This category can be explored through the ‘sweater approach’ taught to me by Rheumatology extraordinaire Dr. Kenny Sunboye. This approach involves multisystem symptoms: As if you were putting on a sweater; think about symptoms involving the hands, arms (and legs), scalp, eyes, nose, mouth, chest, and abdomen, as follows:
Infections and malignancies can be tackled through a system-based approach and screening for ‘B’ symptoms (weight loss and night sweats), with travel history being particularly important concerning infection-related GNs.
Listed below are references for secondary causes for GNs, for when you need a detailed list after a GN has been identified on biopsy – happy hunting!
| GN | Reference for secondary causes: |
|---|---|
| FSGS | J Am Soc Nephrol 2018; 29:759 |
| IgA Nephropathy | Kidney Int 2018; 94:674 |
| Membranous Nephropathy | Seminars in Nephrology 2003; 23:400 |
| MPGN pattern of injury | Pediatr Nephrol 2010; 25:1409 |
| Minimal Change Disease | Clin J Am Soc Nephrol 2017; 12:332 |
With most of the above GNs, the secondary driver is felt to cause the GN directly. For instance, FSGS can manifest as a maladaptive response to a reduction in nephron mass for arising from a variety of causes (Nat Rev Nephrol 2015; 11:76), immunogenic antigens produced by malignancies are thought to mediate secondary membranous nephropathy (Kidney Int 2006; 70: 1510), and liver cirrhosis may drive IgA nephropathy due to failure of Kupfer cells to clear circulating IgA (Nephrol Dial Transplant 1999; 14: 2279).
However, many of these ‘secondary causes’ may simply be associations. The extent to which the secondary cause drives the disease needs to be carefully considered in the correct clinical context, with a multi-disciplinary team’s support in less straightforward cases.
Section 2: Detecting renal involvement in systemic diseases – how do we do it?
Can glomerular involvement drive poorer outcomes in systemic diseases?
In section 1, we explored the importance of searching for secondary causes in confirmed or suspected GN cases. Conversely, detecting glomerular involvement in systemic disease is equally, if not more important. The second part of this report will explore this further.
Glomerular involvement in systemic disease confers significantly poor outcomes for patients. Let’s take three common systemic diseases with glomerular involvement:
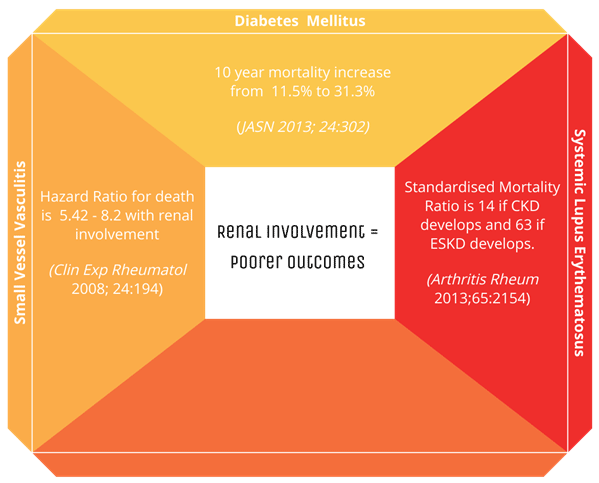
As the diagram above demonstrates, renal involvement confers worse outcomes, and as we will explore in future sessions, it can prompt a change in management.
What’s the best way to screen for glomerular involvement in systemic disease?
The most common method of screening for glomerular involvement is a urine dipstick. How effective is it? Let’s take a look at the sensitivities and specificities of the dipstick (Saudi J Kidney Dis Transpl. 2009;20(3):443)(J Nephrol. 2005; 18: 703):
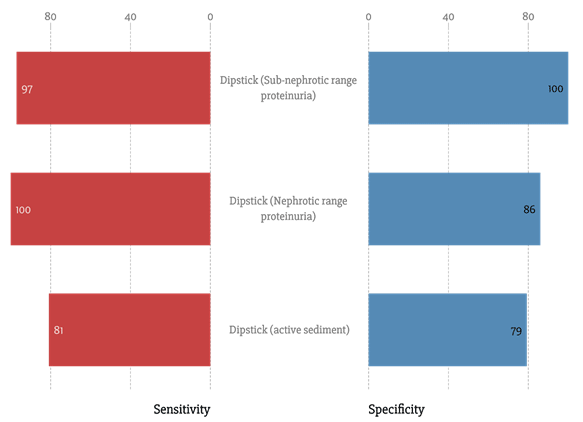
As a reminder, sensitivity is how often the test is positive when it should be, and specificity is how often the test is negative when it should be.
So, it is very good as a screening test. Of course, the urine dipstick can generate false negatives and false positives (more detailed information here: Am Fam Physician 2005; 71:1153). However, in the context of proteinuria, this can easily be verified with formal quantification. Although the gold standard for quantification is a 24-hour urinary collection, this can be cumbersome for patients and is rarely done. Urine protein to creatinine ratio is used far more commonly in practice, although it can be subject to significant day-to-day variability (Am J Kidney Dis. 2012;60(4):561-6). Indeed urine protein creatine ratios have been found to generally correlate poorly with 24-hour urinary collections in the context of glomerular disease, but corrective equations have been proposed to provide a more practical means of quantifying proteinuria, especially in out-patient settings (Kidney Int 2016; 90:1080), where they are the mainstay of urine protein quantification.
Other biomarkers for systemic diseases with glomerular involvement – how valuable are they?
For completion, let us look at the sensitivities and specificities of other serological tests we might order to investigate certain GNs:
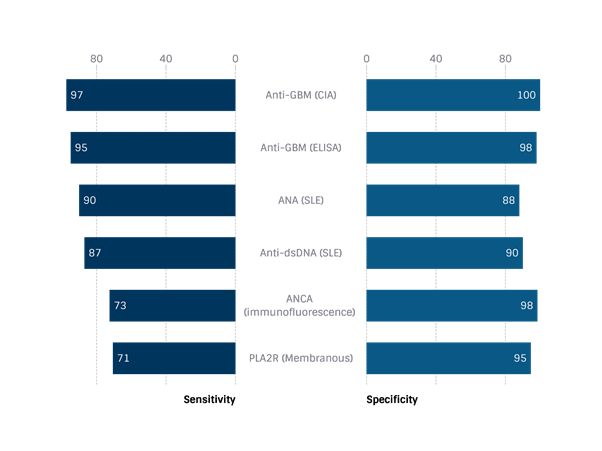
(Kidney Int 1998; 50: 796), (Renal Failure 2019; 42:50), (Lupus 2009; 18: 1276), (Lupus 2009; 18: 1276), (J Nephrol. 2018; 31: 271)
Question 8: How useful are creatinine-based eGFRs in glomerular disease?
While the various measure of proteinuria serves as proxy markers of glomerular injury, we still heavily rely on creatinine-based eGFRs to monitor renal function, influencing critical decisions including initiating therapy in some cases and inclusion in clinical trials.
Several formulas have been proposed to estimate glomerular filtration rates from serum creatinine. However, the most commonly used are CG, MDRD, and CKD-EPI.
The Cockcroft Gault formula is mainly used in pharmacotherapy, producing a ‘creatinine clearance’ in the strictest sense, not an eGFR. Although widely used, it must be noted that the cohort the formula is derived from were mainly young and male and was were in-patients from a single center. The variables included in this equation are age, weight, sex, and serum creatinine (Nephron 1976; 16:31). It has not been validated as a tool for detecting endpoints in glomerular disease.
The MDRD formula was a product of a much larger multicentre study investigating the effects of dietary protein restriction on CKD progression. It involved a larger cohort, from whom GFR was calculated using exogenous markers, and creatinine clearance was estimated from using 24-hour urine collections (Ann Intern Med 1999; 6:461). An eGFR is easier to calculate as only sex, serum creatinine, age, and ethnicity are needed as variables, and indeed it was the recommended equation used by laboratories internationally. However, it did have drawbacks; it was developed from a cohort with CKD, and so is less accurate in patients’ relatively preserved renal function, where it tends to underestimate renal function (J Am Soc Nephrol 2003; 10:2573). Furthermore, it only factors in Caucasians and African-American ethnicities.
The CKD-EPI formula is the most recent widely adopted formula proposed and was developed to address some of the shortcomings of the MDRD equation, and indeed is more accurate at estimating GFRs when renal function is more preserved (Ann Intern Med 2009; 150:604).
Despite the above, all these equations still carry the pitfalls of using a creatinine-based eGFR method, these include:
- Creatinine-based eGFRs are reliant on a constant muscle mass, which can vary with diet (protein consumption) and muscle mass, which can fluctuate between consultations.
- Some medications interfere with creatinine secretion and can therefore disturb steady states
- Creatinine is subject to tubular secretion. The proportion of urinary creatinine that is present as a result of filtration vs. secretion may vary unpredictably over the natural course of a glomerular disease
Furthermore, creatine based eGFRs can be inaccurate in the context of proteinuria, with a potential to overestimate GFR if hypoalbuminemia is present (Nephrol Dial Transplant 2005; 20:707). A more detailed insight into the pitfalls of using serum creatinine to estimate GFR can be found at this reference (J Lab Precis Med 2018;3:71).
Despite these drawbacks, creatinine-based eGFRs still have real validated value in various glomerular diseases. A low creatinine-based eGFR at diagnosis has been validated as a predictor of poor prognosis in GNs, including IgA nephropathy (Nephrol Dial Transplant. 2003;18(8):1541-8).
Trends in creatinine-based eGFRs can also be of great value in assessing patients and their risk of progressive kidney disease. The eGFR slope has been proposed and accommodated as a valid endpoint for clinical trials that include glomerular diseases to facilitate the recruitment of patients with early disease in whom treatment effects might otherwise be missed (J Am Soc Nephrol. 2019;30(9):1746-55).
The limitations of current creatinine-based GFR estimating equations is also discussed in this post: https://pubs.glomcon.org/shortcomings-of-the-current-gfr-estimating-equations/
Given the issues with creatinine-based eGFRs, are there any widely considered alternatives?
Cystatin C is an endogenous marker that has been proposed as an alternative to creatinine for the calculation of estimated GFRs. It’s a low molecular weight ubiquitous protein produced by all nucleated cells, and so it felt to be less variable than creatinine (Biochem J 1990;268:287). It is of interest as an endogenous marker for eGFR as the kidney nearly completely filters it. Although it is almost completely reabsorbed at a tubular level, it is also completely metabolized at this stage and not returned to circulation (Biochem Biophys Res Commun 2007; 357: 1130–1134).
The CKD-EPI Cystatin C equation uses serum Cystatin C, age, and sex to estimate GFR (N Engl J Med. 2012;367:20), and several studies have demonstrated some benefits to using Cystatin C over serum creatinine for calculating eGFR:
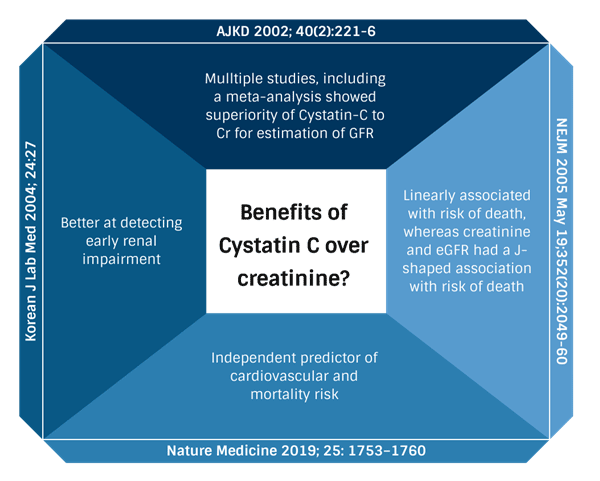
Can Cystatin C based eGFRs add any value to glomerular disease?
The short answer is that it cannot be recommended as beneficial over creatinine-based eGFRs as the data isn’t conclusive. The available information is conflicting, and the test itself is more expensive, so its widespread use cannot be justified currently.
Ultimately a wide variety of single-center, low sample size studies exist, drawing a variety of conclusions, including:
- Cystatin C behaves differently in the context of kidney disease driven by different aetiologies (J Clin Lab Anal. 2018; 32:e22166).
- It is more sensitive than serum creatinine at predicting kidney dysfunction in primary nephrotic syndrome (AJKD 2002; 40(2):221-6)
- Cystatin C-Creatinine combinations may be most accurate (however, the standard used for comparison was a 24-hour urinary creatinine clearance, not an exogenous marker) (Int J Nephrol Renovasc Dis 2015;8:145).
It’s worth noting there are more accurate methods that directly GFR as opposed to estimating it. These are exogenous markers, such as iohexol or inulin. These methods are rarely used due to the added burden they place on patients and the cost, but occasionally are used if a CKD stage diagnosis is called into question or transplant donation is being considered. Given they involve a procedural element, it is unlikely that exogenous methods of measuring GFR will become the standard of care.
Key take-home messages:
- Secondary GNs are common and should be investigated for
- Renal involvement in systemic disease confers a poor prognosis
- The urine dipstick is a cheap and valuable screening tool for detecting glomerular disease
- Despite its drawbacks, creatinine-based eGFRs are the mainstay of glomerular filtration measures and are particularly valuable as a ‘slope’ or trend.
Image credits for the four-panel image (autoimmune, malignancy, infections, and drugs):
Immunity by Lima Studio from the Noun Project
Cancer by Adrien Coquet from the Noun Project
Bacteria by Ian Rahmadi Kurniawan from the Noun Project
Drug by tezar tantular from the Noun Project
