DISEASE MECHANISM
Genetic Focal Segmental Glomerulosclerosis (FSGS)

Dr. Marco Bonilla
Nephrology Fellow
Division of Kidney Disease and Hypertension,
Donald and Barbara Zucker School of Medicine at Hofstra/Northwell
What is the current clinicopathologic classification of focal segmental glomerulosclerosis (FSGS)?
The classification of patients with FSGS lesions is often challenging due to the broad spectrum of underlying causes, our limited understanding of the pathophysiology, and the poor correlation between histopathology and treatment response and clinical outcomes. Therefore, many experts suggest a clinicopathological approach rather than a morphological approach to FSGS classification (morphometrics school of thought vs. pathomechanism-based school of view). The classification into pathomechanism-based (e.g., underlying pathophysiology) categories have been proposed by Dr. Helmut Rennke, Dr. Jeffrey Kopp, and others and discussed in detail during one of the most widely watched GlomCon seminar (https://youtu.be/2kN5MpTOcG8) and most commonly referenced articles on this topic (https://doi.org/10.2215/CJN.05960616).
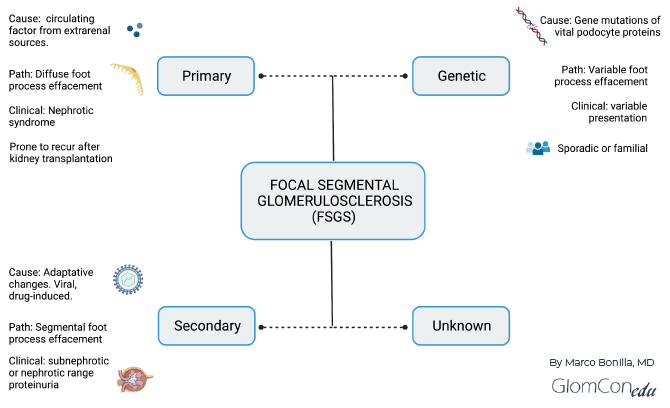
As reviewed by De Vriese et al. (https://doi.org/10.1681/ASN.2017090958), a classification based on clinicopathological assessment will improve clinical management and further guide the design and implementation of clinical trials. The importance of a clinicopathologic classification is based on the different clinical presentations, management, and prognostic implications of the various disorders, all presenting as “FSGS” on histopathology. A clinical practice solely based on histopathological injury patterns is insufficient to predict treatment response, and patients could undergo needless immunosuppression. The KDIGO 2021 guidelines adopted this proposed pathomechanism-based approach to FSGS classification, differentiating between primary (immune medicated), genetic, secondary, and undetermined causes (https://doi.org/10.1016/j.kint.2021.05.021). Figure 1 summarizes this classification.

Figure 1. Summary of clinicopathological classification of FSGS based on the underlying cause.
A frequent question: “When is it appropriate to perform genetic testing in patients with FSGS?”
In a cohort of patients with steroid-resistant nephrotic syndrome, underlying genetic mutations were identified in 24% of early childhood (4-12 months of age), 36% of late childhood (13 months to 5 years of age), 25% of adolescence (6-12 years of age), and up to 14% of adult FSGS patients (https://doi.org/10.2215/CJN.05260610).
With the rapid advancements in identifying numerous genetic causes of podocytopathies and glomerular diseases that present as FSGS on the biopsy, it is becoming more apparent that genetics play an important role in differential diagnosis and workup of FSGS. Although routine genetic screening is not recommended for all patients with FSGS lesions, it should be considered in particular cases.
Below is a proposed algorithmic approach to genetic testing in FSGS adopted from De Vriese et al. (https://doi.org/10.1681/ASN.2017090958). In addition, a family history of chronic kidney disease or nephrotic syndrome should alert physicians to a possible genetic cause. Determining a genetic cause of FSGS could avoid unnecessary treatments in pre-and post-transplant patients.
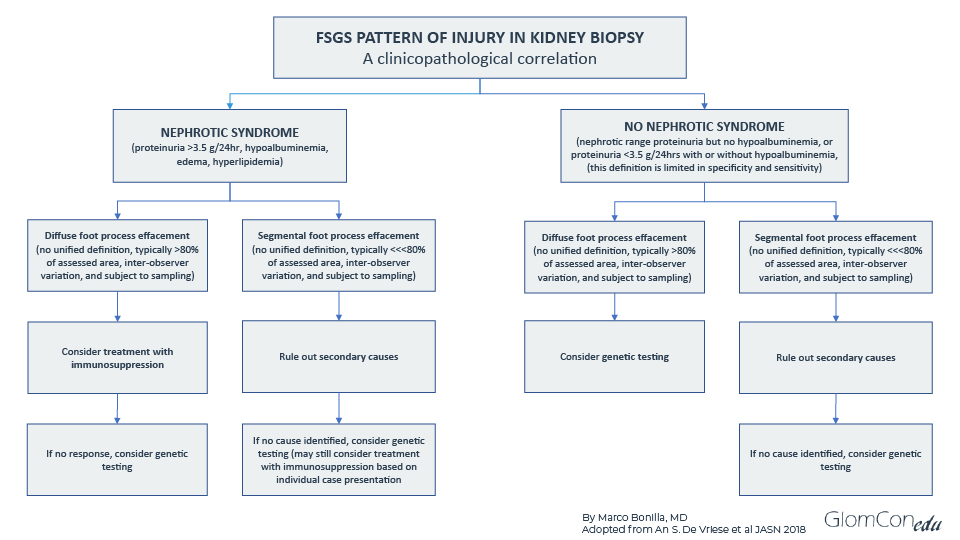
Figure 2. An algorithmic approach to genetic testing in FSGS patients.
What are the associated genes, and how is the clinical manifestation in genetic FSGS?
Genome-wide association studies (GWAS) are used to identify genotype-phenotype associations within the genomes of many individuals. In nephrology, GWAS has been used to identify genetic variants contributing to the development of kidney diseases, for example, FSGS and APOL1 in patients of African ancestry. GWAS identified a susceptibility locus on chromosome 22 that codes for two variants in the APOL1 gene (G1 and G2), allowing an increased risk for developing FSGS (https://doi.org/10.1126/science.1193032 ). Genetic causes of FSGS can be categorized into renal-limited and syndromic, the latter being associated with extra-renal manifestations such as hearing loss and vision impairment. Some of the gene mutations causing renal-limited and syndromic FSGS are reviewed by De Vriese et al. (https://doi.org/10.1681/ASN.2017090958). NPHS1 (nephrin) and NPHS2 (podocin) are the most commonly affected genes in early-onset cases. In adulthood, NPHS2 (podocin) and IFN2 (a member of the formin family of actin-regulating proteins) comprise the most common culprits (https://doi.org/10.1093/ckj/sfx143 ). Genetic FSGS can present in childhood or adulthood depending on the affected gene and manifest with variable degrees of proteinuria and foot process effacement. In some cases of genetic susceptibility (e.g., APOL-1 mutation), the clinical manifestation only occurs after the exposure of “second hit” events such as chemical toxicity, inflammation, or infection.
Is there any treatment for Genetic FSGS?
The majority of the genetic mutations are identified in cases of steroid-resistant nephrotic syndrome (SRNS), and unfortunately for the vast majority of these mutations, currently, there are no specific therapies. However, in certain subgroups of genetic FSGS, treatments targeting the underlying cofactor deficiencies can improve clinical outcomes. For instance, early replacement of CoQ10 can reduce proteinuria in patients with genetic mutation of CoQ6 monooxygenase (https://doi.org/10.1007/s00467-020-04914-8 ). Another underlying gene mutation that could be treated with vitamin replacement is the CUBN gene that encodes cubilin. These patients have a hereditary form of megaloblastic anemia due to vitamin B12 deficiency and childhood-onset SRNS and may benefit from Vitamin B12 replacement (https://doi.org/10.1681/ASN.2011040337 ). A phase 2 trial for patients with APOL1 mediated FSGS, investigating a new drug (VX-147) that inhibits APOL1 protein has shown positive results. VX-147 added to the standard of care regimen (ACE/ARB + immunosuppressant or low dose corticosteroids) for 13 weeks showed a substantial reduction in proteinuria of about 47.6% compared to baseline (https://news.vrtx.com/press-release/vertex-announces-positive-results-phase-2-study-vx-147-apol1-mediated-focal-segmental).
With the advances in genetic testing and the discovery of many more genes associated with FSGS, a correct differential diagnosis requires a clinicopathologic approach, prompting physicians to pursue a thorough evaluation in each patient to adequately identify and determine the underlying cause of an FSGS pattern of injury. Distinguishing between the subclasses of FSGS (primary, secondary and genetic forms) is essential to guide the treatment approach and to design new clinical trials in developing targeted therapies for genetic FSGS.

Excellent clinical approach
Thanks intresting