TREATMENT
Lupus Nephritis Treatment – What is Next?

By Dr. Sayali B. Thakare
Assistant Professor
Seth GSMC and KEM Hospital
Mumbai, India
GlomCon Editors involved in development of this article: Paolo Nikolai So, Sonia Rodriguez, Mohamed Hassanein, Edgar Lerma, and Nasim Wiegley
Introduction
Lupus Nephritis (LN) develops in 40 to 60% of patients with systemic lupus erythematosus (SLE). The renal domain of SLICC/ACR (Systemic Lupus International Collaborative Clinics/American College of Rheumatology) Damage Index (SDI) is associated with early mortality in SLE. Hence, preventing kidney damage in SLE has long-term prognostic implications. Currently used standard of care (SOC), corticosteroids in combination with broad-spectrum immunosuppressants, has dramatically improved patient survival in LN (from 17% at 5 years in untreated patients to 80% in those treated with SOC), though not without the burden of drug toxicities. Yet, 10 to 30% of patients with LN progress to end-stage kidney disease (ESKD) within 15 years. This article provides an overview of emerging therapies for LN and major randomized controlled trials (RCTs) involved in drug development.
Novel Therapeutic Targets for Lupus Nephritis
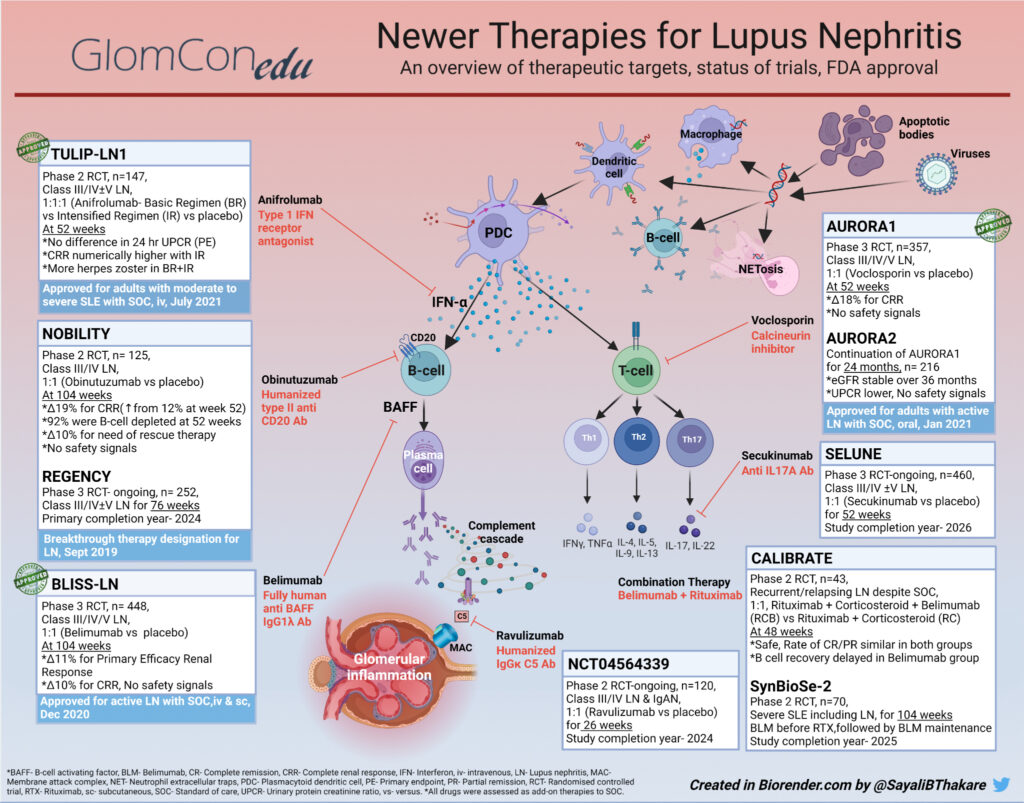
The diversity of immune responses in SLE and LN provides many attractive biologic targets (Figure 1). Plasmacytoid dendritic cells (PDCs) are activated by signals from other immune cells reacting to self-antigens. Interferon-alpha (IFN-α) released from activated PDCs serves as an amplifier to major axes of immune activation. PDCs stimulate the production of other antigen-presenting cells. They upregulate Major Histocompatibility Complex II (MHC II) and co-stimulatory molecules leading to the activation of T-cells. CD4 helper T (TH) cells, thus activated, produce a repertoire of cytokines promoting autoreactive B-cell differentiation to plasma cells. Plasma cells further drive higher auto-antibody expression and immune complex formation in SLE and LN.
Delving into candidate drugs targeting these pathways is arduous. A comprehensive study of clinical trials in LN registered with ClinicalTrials.gov enlists as many as 126 RCTs initiated between 1998 and 2020, including those for 27 types of biological agents. Amongst these, anti-inflammatory agents act acutely by limiting immunological damage, making them useful as induction agents. Therapies that target autoimmunity work towards attenuating disease activity and thus prevent accruing damage from repeated flares.
Despite tremendous progress in unraveling the pathobiology of LN, only a handful of drugs have shown meaningful benefits with acceptable side-effect profiles in phase 2 and 3 clinical trials (Table 1). Some of these agents have received approval for use by regulatory agencies (Figure 1). Belimumab, an anti-BAFF monoclonal antibody, was the first biological agent approved for SLE in 2011. After a series of focused trials in patients with renal involvement, Belimumab was subsequently approved for LN in December 2020. Voclosporin, a novel oral calcineurin inhibitor (CNI), was approved for LN in January 2021. The most recent addition to the armamentarium of SLE is Anifrolumab, an anti-IFN-α (interferon-alpha) monoclonal antibody (mAb). Anifrolumab was approved for moderate to severe SLE in August 2021, making it the only new drug for SLE in over a decade. Anifrolumab is currently undergoing a phase 3 trial in LN (Table 2). Noting previously concluded trials helps gain perspective about the impact of a given drug action on the pathogenic pathways in LN. Table 1 outlines an updated list of concluded RCTs that either failed to meet the primary endpoint, showed unacceptable drug toxicity or were terminated prematurely.
Table 1. Terminated Randomized Controlled Trials in Lupus Nephritis and Clinical Outcomes
| Drug | Mechanism of action | Trial registration | Phase | Status |
|---|---|---|---|---|
| Trials with favorable outcomes | ||||
| Filgotinib/ Lanraplenib | Small molecule inhibitor of JAK1/ ATP- competitive inhibitor of spleen tyrosine kinase (SYK) | NCT03285711
(Class V LN) |
2 | Median reduction of 50.7% in proteinuria at 16 weeks for Figlotinib, no benefit with Lanraplenib |
| Narsoplimab (OMS721) | MASP-2 inhibitor mAb (lectin pathway inhibitor) | NCT02682407 | 2 | 69% reduction of proteinuria in 4/5 patients |
| Trials with unfavorable outcomes | ||||
| B-cell Therapies | ||||
| Rituximab | Anti CD20 mAb | NCT00282347
(LUNAR) |
3 | Failed to meet the primary endpoint, no safety signals |
| Ocrelizumab | Anti CD20 mAb | NCT00626197
(BELONG) |
3 | Early termination due to higher incidence of serious infections. Failed to meet primary endpoint. |
| Atacicept | Fusion protein between TACI and Fc portion of IgG | NCT00573157 (APRIL-LN) | 2/3 | Terminated early owing to severe infective complications and hypogammaglobulinemia |
| Blisibimod | Binds to BAFF and prevents interaction with BAFF receptors | NCT02514967
(CHABLIS7.5) |
3 | Terminated due to failure of prior trial CHABLI SC1 |
| Bortezomib | Proteasome inhibitor | NCT01169857 | 4 | Withdrawn as the previous RCT in SLE did not meet primary endpoint and had higher adverse effects |
| Ixazomib citrate | Proteasome inhibitor | NCT02176486 | 1 | Terminated due to insufficient enrolment, no safety concerns |
| Co-stimulatory Pathways | ||||
| Abatacept | Fusion protein binding to CD80/86 resulting in blockade of CD28 co-stimulation | NCT00774852
(ACCESS) NCT01714817 (ALLURE) |
2
2/3 3 |
Failed to meet the primary endpoint, no safety signals
Failed to meet the primary endpoint, no safety signals Failed to meet the primary endpoint, no safety signals |
| BI-655064 | Anti-CD 40 mAb (Co-stimulatory blockade) for maintenance therapy | NCT02770170 | 2
2 |
Effect size 15.2% and 9.1% for 120-180 mg dose at 52 weeks |
| Dapirolizumab Pegol (CDP7657) | PEGylated anti CD40 Ab fragment | NCT02804763 | 2 | Failed to meet primary endpoint, well tolerated, smaller risk of thromboembolic events |
| Ruplizumab (BG9588) | Anti CD40L mAb (co-stimulation blocker) | NCT00001789 | 2 | Terminated due to thrombo-embolic events |
| Cytokine Targeted Therapies | ||||
| AMG-811 | Anti IFN-gamma | NCT00818948 | 2 | Favorable safety profile and PK but no clinical impact |
| BIIB023 | Human mAb against TWEAK | NCT01499355 (ATLAS) | 2 | Prematurely terminated, no clinical efficacy |
| Sirukumab (CNTO-136) | Human IgG1k IL-6 mAb | NCT01273389 | 2 | Prematurely terminated, no increased efficacy nor acceptable safety profile, AE- mostly infections |
| Ustekinumab | Anti- IL-17/23 mAb | NCT03517722
(LOTUS) |
3 | Terminated in June 2020 due to lack of efficacy in interim analysis |
| Others | ||||
| Abetimus sodium | Tolerogen- reduces production of dsDNA antibodies | NCT00089804
(ASPEN) |
3 | Terminated due to lack of efficacy |
| Deucravacitinib (BMS-986165) | Selective tyrosine kinase Tyk-2 inhibitor selectively blocking the IL-17/23 and IFN type I p/w | NCT03943147 | 2 | Terminated due to insufficient enrolment |
Remarkably, not all drugs demonstrating efficacy in SLE display replicable results in LN. The role of local immune pathways, such as the intra-renal inflammatory cycle, interstitial lymphocytic aggregates, or germinal centers within the kidneys, may be implicated. Therapeutic categories of drugs evaluated in trials for patients with LN, such as B-cell therapies and co-stimulatory blockade agents, are described.
(1) B-cell Directed Therapies
B-cells have a central role in the pathogenesis of LN and are the most investigated axis for drug therapy. Approaches include B-cell depletion (i.e., Rituximab, Obinutuzumab, Ofatumumab, Ocrelizumab), anti-B-cell activation (i.e., Belimumab, Obexelimab, Atacicept, Blisibimod, Ianalumab), co-stimulatory blockade (i.e., Iscalimab, Abatacept, Ruplizumab, Dapirolizumab pegol), and anti-plasma cell therapy (i.e., Bortezomib, Daratumumab, Ixazomib). Obinutuzumab, a humanized anti-CD20 monoclonal antibody (Figure 1), achieves higher and more sustained B-cell depletion than Rituximab and received breakthrough therapy designation from FDA for LN in 2019 based on the results of the NOBILITY trial. However, BAFF levels increase with B-cell depletion. Therefore, the sequential use of Belimumab with Rituximab in the SynBioSe-2 trial (Figure 1) is evaluating synergistic inhibition of the repopulation of autoreactive B-cells. Long-lived autoreactive plasma cells are found in the circulation and renal interstitium and play a role in flares; hence the rationale for using plasma cell-depleting agents in LN. Bortezomib, a proteasome inhibitor, caused significant neurotoxicity in patients with LN leading to the early termination of the trial (Table 1). Finally, an immuno-proteosome inhibitor (proteasome inducible by IFN-gamma), KZR 616, is currently being investigated with encouraging interim results.
(2) Co-stimulatory Blockade Agents
CD40/CD40L and CD28/CD80/86 are attractive pathways for therapeutic targets in immune-mediated disorders. A trial involving an anti-CD40L antibody, Ruplizumab, was halted due to severe thromboembolic events. A PEGylated anti-CD40 Ab fragment, Dapirolizumab Pegol (CDP7657), was tested in LN with an attenuated risk of thromboembolic events; however, the trial did not meet the primary endpoint. Due to potential benefits, nevertheless, a phase III RCT is in progress.
Abatacept, a CD28/CD80 pathway blocker, failed to meet the primary endpoint in three RCTs. Since no safety signals are reported and being an agent affecting autoimmunity, Abatacept might work better as maintenance therapy. BI 655064 and Iscalimab, both humanized anti-CD40 mAbs, are also therapeutic candidates currently under investigation.
(3) Other Potential Target Therapies in LN
These include but are not limited to, cytokine-directed therapies (i.e., Ustekinumab, Sirukumab, BIIB023, AMG 811, Secukinumab, Guselkumab), anti-complement therapies (i.e., Ravulizumab, APL-2, Iptacopan, Narsoplimab) and miscellaneous (i.e., kinase inhibitors, Fc receptor antagonist, immune-proteasome inhibitor). Itolizumab, anti-CD6 mAb, received fast-track designation from the FDA for LN in December 2019. Most of these agents showed efficacy in previous studies in SLE and are now being investigated for LN. Table 2 enlists trials that are currently ongoing and are expected to be completed in the near future. As we eagerly wait for the results of these trials, optimizing the current broad-spectrum therapy assumes prime importance. Steroid reduction, a strategy incorporated into the current guidelines of SOC, is one way toward making therapy in SLE and LN safer.
Table 2. Ongoing Randomized Controlled Trials of Newer Agents in Lupus Nephritis
| Drug | Mechanism of action | Trial registration | Phase | Participants (n) | Timeline |
|---|---|---|---|---|---|
| B-cell Therapies | |||||
| Daratumumab | Anti CD38 mAb | NCT04868838 | 2 | 12 | 2021-2024 |
| Ianalumab (VAY736) | Anti B-cell activating factor (BAFF) receptor mAb | NCT05126277 | 3 | 420 | 2022-2031 |
| Obinutuzumab | Anti CD20 mAb | NCT04221477 | 3 | 252 | 2020-2028 |
| Co-stimulatory Blockade | |||||
| Iscalimab (CFZ533) | Anti CD40 mAb | NCT03610516 | 2 | 75 | 2018-2023 |
| Cytokine targeted therapies | |||||
| Guselkumab | IL-23 p19 subunit inhibitor | NCT04376827
(ORCHID-LN) |
2 | 60 | 2020-2025 |
| Secukinumab | Anti IL17A mAb | NCT04181762 (SELUNE) | 3 | 460 | 2020-2026 |
| Complement Therapies | |||||
| APL-2 (Pegcetacoplan) | Binds and inhibits cleavage of C3 into C3a & C3b | NCT03453619
DISCOVERY |
2 | 21 | 2018-2022 |
| Iptacopan (LNP023) | Small molecule Factor B inhibitor | NCT05268289 | 2 | 240 | 2022-2028 |
| Ravulizumab | Anti C5 IgG k mAb | NCT04564339 | 2 | 120 | 2020-2024 |
| Others | |||||
| Anifrolumab | Anti IFN- | NCT05138133 | 3 | 360 | 2022-2025 |
| Itolizumab | Anti CD6 mAb | NCT04128579
(EQUALISE) |
1b | 55 | 2019-2023 |
| KZR-616 | Selective inhibitor of LMP7 & LMP2 (immune-proteosome) | NCT03393013
(MISSION) |
1b/2 | 39 (LN-2) | 2018-2022 |
| Nipocalimab | Fc receptor antagonist (increases degradation of IgG) | NCT04883619 | 2 | 80 | 2022-2026 |
| Sirolimus | mTOR inhibitor | NCT04892212
(Single centre) |
2 | 20 | 2021-2023 |
| Zanubrutinib | Bruton’s tyrosine kinase (BTK) small molecule inhibitor | NCT04643470 | 2 | 200 | 2020-2023 |
| Belimumab + Rituximab | Combination therapy | NCT03747159 (SynBioSe-2) | 2 | 70 | 2018-2025 |
Conclusion
The armamentarium of SLE is rapidly expanding. However, despite the steady engagement of resources from basic sciences and clinical medicine, there is a long way to go. Successful drug development often unfolds over many years. In order to transform clinical care globally, benefits from scientific discoveries need to be made widely available and affordable, which assumes the next level of the challenge after the success of a clinical trial. Enthusiasm toward emerging therapies is tempered by a realization that current research focuses heavily on induction agents. More studies are warranted for specific situations like membranous LN, childhood-onset LN, maintenance therapy, and antiphospholipid antibody syndrome. Precision medicine, matching a given drug to the most responsive disease phenotype, appears to be the future in LN (e.g., Anifrolumab in SLE patients with a high IFN signature). Yet like never before, we might be poised to have plenty more in the immediate future.

Saludos, soy el doctor Daniel, nefrólogo pediátrico de Cuba, me parece muy oportuna su actualización, en mi hospital tengo varios pacientes con nefropatía lúpica, entre ellos una niña de 17 años y un niño de 15, lamentablemente no contamos con estás terapéuticas novedosas.
It has been a marvellous actualization of the new targets to Lupus andLN.Thant you
Thank you for appreciating this.🙏🏻
Thank you
Nice
Thank you.🙏🏻