MINI-REVIEW
Nerve Epidermal Growth Factor-like 1 (NELL-1) in Membranous Nephropathy

By Dr. Subashri Mohanasundaram
Assistant Professor
Department of Nephrology
Government Thoothukudi Medical College & Hospital, India
Introduction
Membranous nephropathy (MN) is characterized by subepithelial deposition of immune complexes along the glomerular basement membrane, which usually manifests with nephrotic syndrome. Phospholipase A2 receptor (PLA2R – discovered in 2009) and thrombospondin type 1 domain containing 7A (THSD7A – discovered in 2012) are two of the target antigens in 70% and 1-5% of primary MN, respectively. In the remaining cases, the target antigens were unknown until recently. Fortunately, laser microdissection of glomeruli from kidney biopsy samples followed by mass spectrometry enabled the identification of several novel target antigens in PLA2R-negative MN. One such antigen is nerve epidermal growth factor-like 1 (NELL-1).
NELL-1
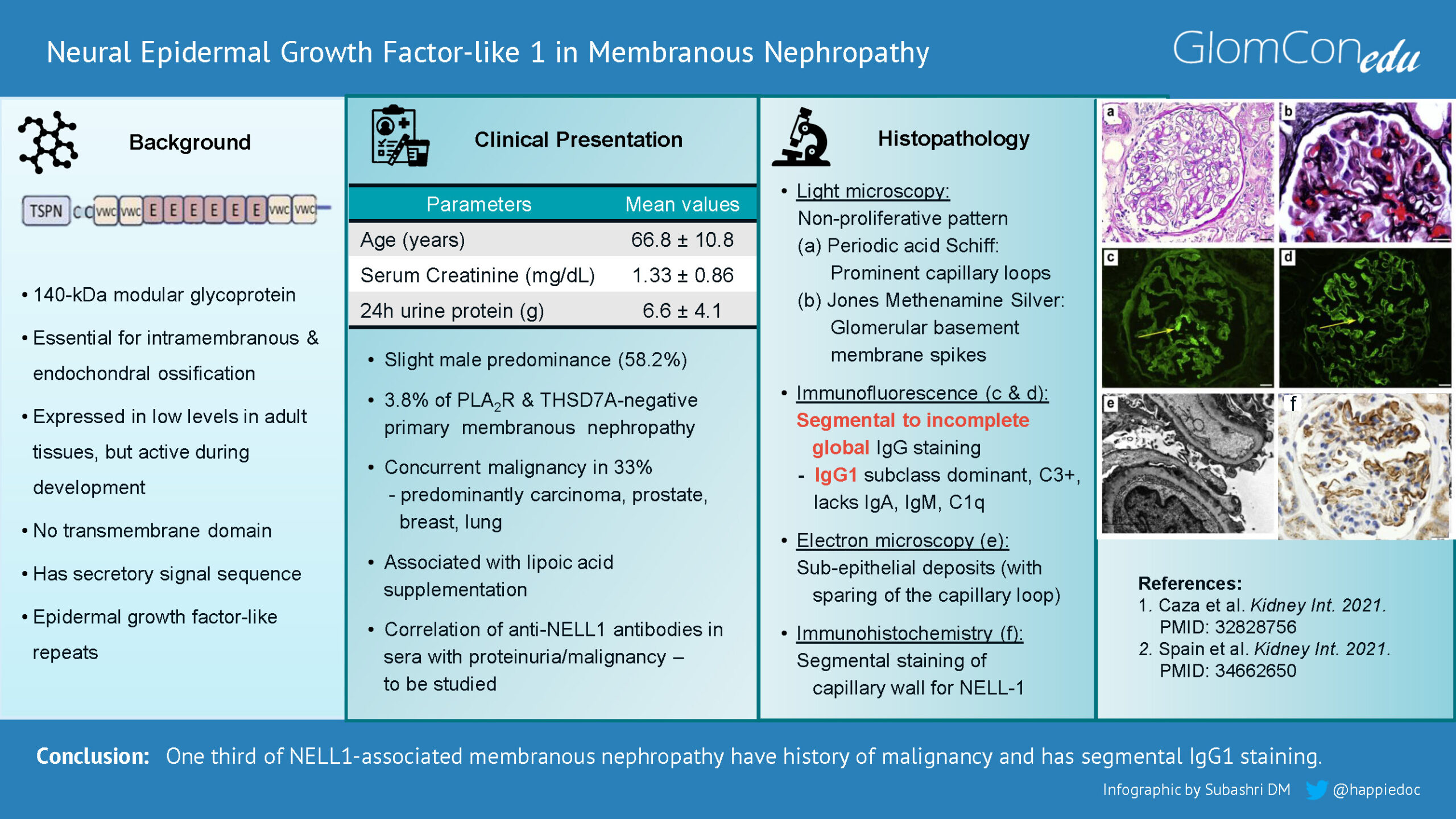
NELL-1 is a gene similar to the ‘Nel’ gene, which encodes a protein with epidermal growth factor-like (EGF-like) repeats strongly expressed in neural tissue. NELL-1 protein is a 140-kDa modular glycoprotein active during development and is highly expressed in osteoblasts. It promotes bone regeneration and is essential for intramembranous and endochondral ossification. The C-terminal region of NELL-1 mediates osteoblastic cell adhesion through integrin α3β2. NELL-1 is overexpressed within prematurely fusing sutures in craniosynostosis, one of the common congenital craniofacial deformities. NELL-1 is also expressed in kidneys, especially in tubules. Although 5 to 25% of glomerular cells express NELL-1 at the mRNA level, the protein is barely detected in the glomeruli. NELL-1 was found to be overexpressed in NELL-1-associated MN.
Clinical Presentation
In 2019, Sethi et al. identified 34 NELL-1 positive cases (16%) out of 210 PLA2R-negative MN biopsies from multiple discoveries and validation cohorts combined. The inference from the combined cohort reveals that NELL-1 positive cases were more than twice the number of THSD7A positive cases in his original study (15 THSD7A positive cases out of 154 PLA2R-negative, 10%). These findings suggest that NELL-1 is the second most common antigen after PLA2R in primary MN.
Subsequently, Caza et al. in 2021 noted that 3.8% of PLA2R and THSD7A negative MN cases were positive for NELL-1 antigen. This cohort found a slight male predominance (58.2%) and a greater association with malignancy (33%). The predominantly associated malignancies were prostate, breast, lung, kidney, and skin carcinoma. The mean age of presentation of NELL-1 positive MN was 66.8+ 10.8 years. This increased age may explain the higher association with malignancy. At the time of biopsy, the mean serum creatinine was 1.33+0.86 mg/dL, and the mean 24-hour urine proteinuria was 6.6+4.1 grams. Similar to anti-PLA2R antibodies, anti-NELL-1 antibodies were found in the sera of these patients. However, the titers of these antibodies and their correlation with proteinuria or underlying malignancy need further evaluation.
Later, a large retrospective cohort from China investigated 832 MN patients. Thirty-five percent of the PLA2R and THSD7A negative cases were positive for NELL-1. This cohort of patients had female predominance, no incidence of malignancy, and rare positivity for serum NELL-1 antibodies compared to the previous studies. Interestingly, one case was double-positive for both NELL-1 and PLA2R.
A brief report published by Spain et al. described four biopsy-proven cases of NELL-1-associated MN following the supplementation of lipoic acid and a fifth suspected case. Lipoic acid is an over-the-counter antioxidant and insulin-mimetic supplement investigated in treating diabetes, schizophrenia, and multiple sclerosis. These patients achieved remission after discontinuation of the offending agent. Lastly, Kudose et al. described a series of 9 MN cases in hematopoietic stem cell transplant recipients, of which two patients were diagnosed with NELL-1 positive MN. This raises the question of whether the NELL-1 positive MN can be associated with other non-malignant conditions.
Histopathology
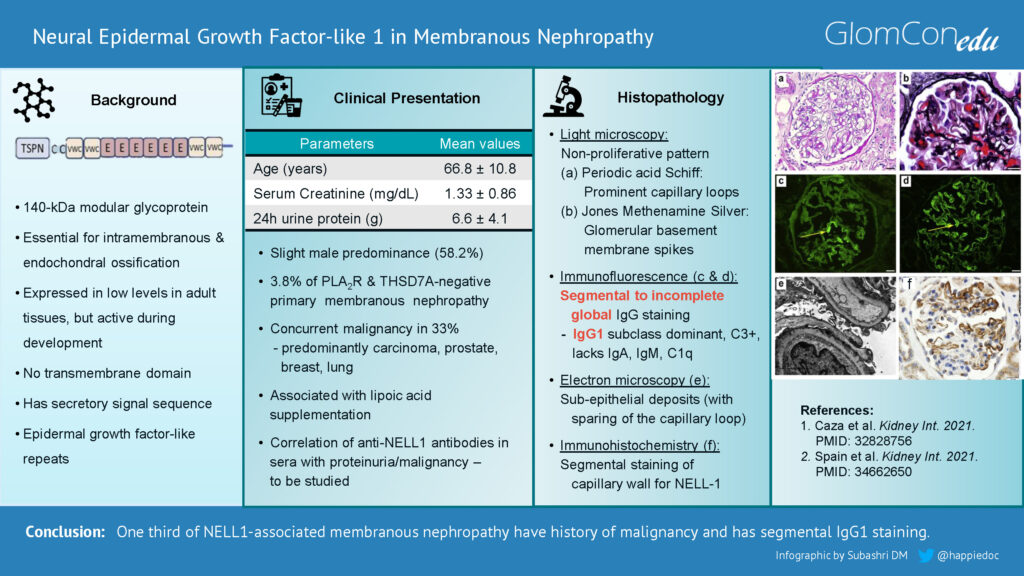
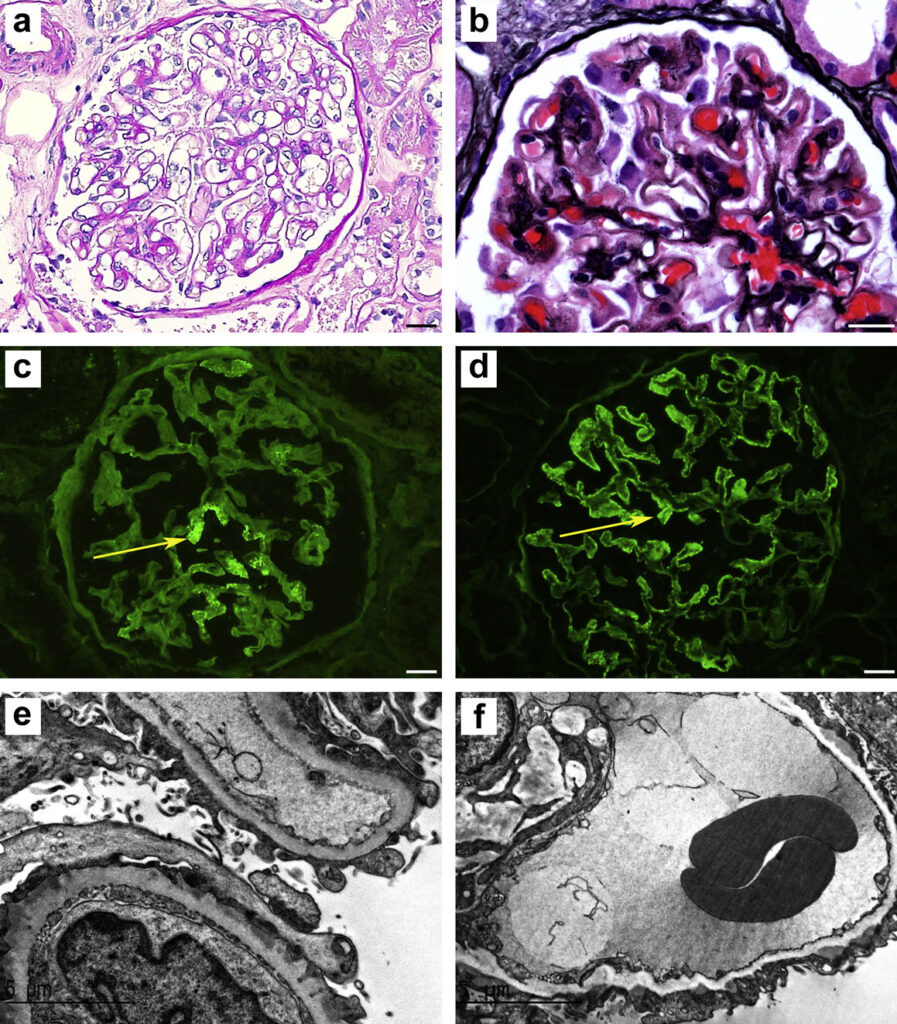
In some aspects, the histopathology of NELL-1-associated MN is unique compared with other forms of MN. Light microscopy features are similar to other forms of MN (Figures 1 & 2). However, immunofluorescence staining in NELL-1-associated MN is unique. In contrast to classical MN, glomeruli show segmental to incomplete global IgG staining, predominant IgG1 subclass positivity, and negative staining for other immune reactants (IgA, IgM, and C1q). Histopathological similarities between NELL-1-associated MN and malignancy-associated MN include IgG1 positivity, segmental sparing of glomerular capillary loops, and the presence of mesangial immune deposits.
Electron microscopy shows the segmental distribution of subepithelial electron-dense deposits, sparing some capillary loops. Immunohistochemistry can demonstrate staining for NELL-1 in the glomerular capillary loops.
Figure 1. Light microscopy: (a) Periodic acid-Schiff showing non-proliferative pattern and prominent capillary loops. (b) Jones methenamine silver stain showing “spikes” along the glomerular basement membrane spikes and holes. (c & d) Immunofluorescence microscopy demonstrating segmental and incomplete global IgG staining in glomeruli. (e) Electron microscopy showing capillary loop sparing of electron-dense immune-type deposits. (f) Immunohistochemical staining for neural epidermal growth factor-like 1 protein (NELL-1) showing segmental positivity. (Images with permission from Caza et al. (2021). 10.1016/j.kint.2020.07.039)
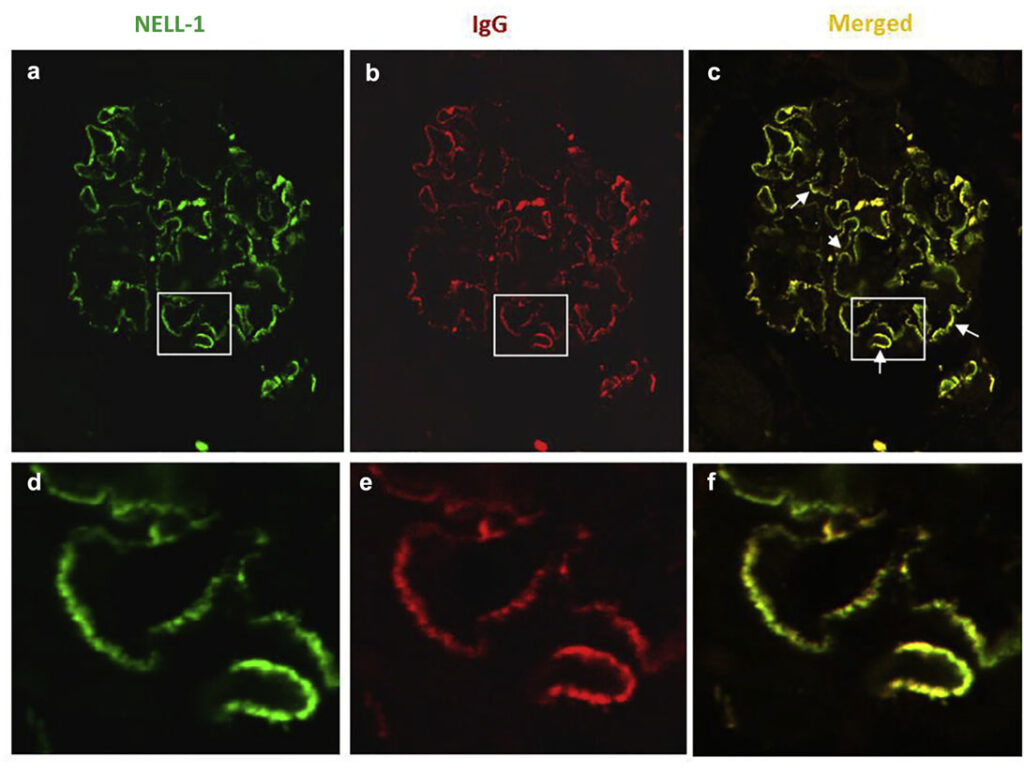
Figure 2. Detection of neural epidermal growth factor-like 1 protein (NELL-1) and IgG in glomerular-immune deposits in NELL-1–associated membranous nephropathy cases by confocal immunofluorescence microscopy analysis. Glomeruli double-labeled with (a) anti-NELL-1 (green) and (b) anti-human IgG (red); (c) the merged image is shown. (Image with permission from Sethi et al. (2020). 10.1016/j.kint.2019.09.014)
Treatment
Currently, the management of NELL-1-associated MN is similar to other forms of MN, with a particular focus on identifying associated malignancy. Supportive treatment would include anti-proteinuric measures by angiotensin-receptor blockers or angiotensin-converting enzyme inhibitors. Upon identifying any associated malignancy, appropriate treatment to manage the underlying cancer may provide remission in these patients.
Concerning immunosuppression, there is only limited observational data from retrospective studies. In a case series of four patients with NELL-1-associated MN from Japan, one patient was treated with steroids alone, and another patient was treated with steroids and cyclophosphamide. Both patients had achieved remission in less than six months. In a Chinese cohort that included 15 NELL-1-associated MN patients, 12 patients were followed up for a period of 25±21 months. None of the patients had any identifiable malignancy. Out of these 12 patients, 10 patients had received immunosuppression in the form of either cyclosporine alone or steroids with cyclophosphamide/cyclosporine. Eleven (92%) patients achieved complete or partial remission, and no patient experienced a relapse. The total remission rate of patients who were isolated NELL-1 positive (92%) was higher than that of patients who were isolated PLA2R positive (62%).
The role of serum anti-NELL-1 antibodies as a biomarker to monitor treatment response and prognosis needs further studies.