DIFFERENTIAL DIAGNOSIS
Proteinuria and Hematuria: Going Beyond the Glomerulus
Acknowledgment: Based on GlomCon’s Glomerular Disease Virtual Fellowship seminar “The Guiding Principles for Evaluation of Glomerular Disorders” by Dr. Richard Glassock.

By Dr. Ayman Al Jurdi
Nephrology Fellow
Massachusetts General Hospital / Brigham and Women’s Hospital

Edited by Dr. Helena Edwards
Post-CCT Nephrology Fellow,
Imperial College Healthcare NHS Trust, London
What are the different etiologies of proteinuria?
The glomerular filtration barrier is composed of three major structures: glomerular endothelial cells, the glomerular basement membrane (GBM) and podocytes. Together, they create a size and charge barrier to the filtration of proteins into the urine. This results in predominantly low molecular weight proteins being filtered across the glomerulus under physiologic conditions. After entering Bowman’s space, the majority of this protein is then reabsorbed in the proximal tubule, resulting in only a very small amount of daily protein excretion in the urine (<150mg/day) as shown in Table 1.
Normal and abnormal degrees of proteinuria as assessed by urine albumin/creatinine ratio (ACR), protein/creatinine ratio (PCR), and urine dipstick. Created by Marina de Cos Gomez. Adapted from KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease.
| Degree of proteinuria | Normal to mildly increased | Moderately increased | Severely increased |
|---|---|---|---|
| ACR | <30 mg/g <3 mg/mmol |
30-299 mg/g 3-30 mg/mmol |
≥300 mg/g ≥30 mg/mmol |
| PCR | <150 mg/g <15 mg/mmol |
150-499 mg/g 15-49 mg/mmol |
≥500 mg/g ≥50 mg/mmol |
| Urine protein dipstick | Negative to trace | Trace to 1+ | 2+ or greater |
Higher levels of proteinuria can result from a variety of pathologies across that pathway. The different etiologies of proteinuria can be classified into five main types:
- Benign proteinuria
- Overflow proteinuria
- Glomerular proteinuria
- Tubular proteinuria
- Post-kidney proteinuria
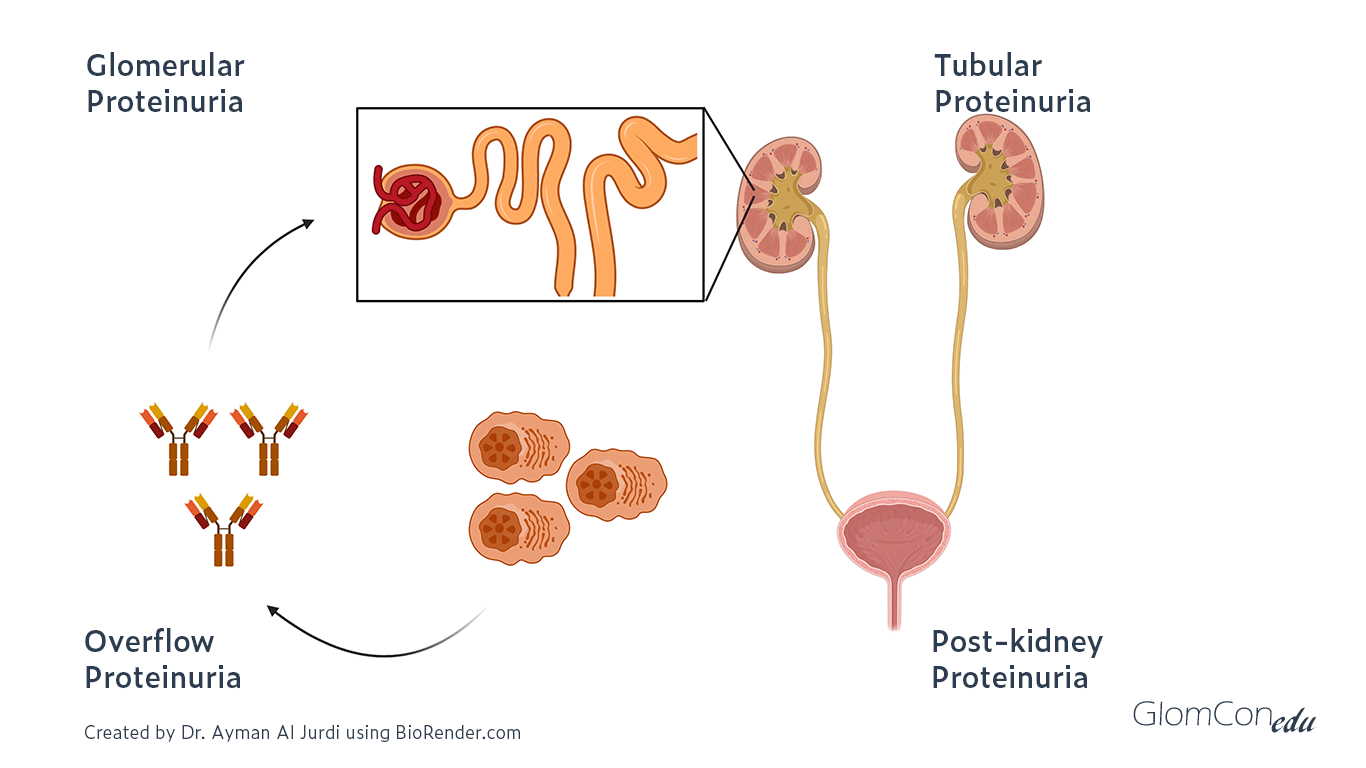
Different etiologies of proteinuria
Benign proteinuria can be seen in the setting of fever, exercise, or orthostatic proteinuria. The pathophysiology is not completely understood, but the proteinuria resolves in patients on subsequent evaluation after the resolution of the etiology (e.g. a few days without fever/exercise). Orthostatic proteinuria is another benign etiology of proteinuria where protein excretion is elevated in the upright position but is normal in the supine position.
Overflow proteinuria is due to the increased production of low-molecular-weight proteins that are then filtered across the glomerulus. The predominant type of protein in the urine depends on the underlying process. A classic example is light chain proteinuria in the setting of multiple myeloma.
Glomerular proteinuria usually results from damage to podocytes, the GBM, or glomerular endothelial cells. Glomerular proteinuria tends to be predominantly albuminuria (UACR/UPCR usually >40%) and the magnitude of proteinuria is variable but can reach >10g per day.
Tubular proteinuria results from tubulointerstitial disease impairing proximal tubular reabsorption of low-molecular-weight protein, as can be seen in hemodynamic acute tubular injury, tubulointerstitial nephritis or Fanconi’s syndrome in the setting of multiple myeloma. Tubular proteinuria typically results in non-albumin predominant proteinuria (UACR/UPCR usually <40%) and the magnitude of proteinuria is usually <2g/day.
Post-kidney proteinuria can be seen in patients with inflammation of the urinary tract such as in nephrolithiasis, urinary tract infections, and tumors along the urinary tract. Typically the proteinuria is <1g/day.
What are the limitations of using the dipstick to assess proteinuria in glomerular disease?
While urine dipstick assessment for protein is cheap and can be done as a point-of-care (POC) test, there are several limitations to using it to assess proteinuria:
- It is highly dependent on the concentration of urine, leading to over or underestimation of proteinuria
- It predominantly detects albuminuria, therefore can miss other types of proteinuria such as light chain proteinuria
- It can miss lower levels of albuminuria, which can result in CKD misclassification and affect treatment
- Protein excretion varies throughout the day potentially affecting the result
- False positives can be seen in patients with alkaline urine (pH ~8) and those who have recently received iodinated contrast
Should spot or 24-hour collections be used for quantification of proteinuria/albuminuria in the diagnosis and follow up of patients with glomerular disease?
Both spot and 24-hour collections can be used in the diagnosis and follow-up of patients with glomerular disease, with each having its advantages and limitations.
Comparison of urine dipstick, 24-hour excretion rate, albumin/creatinine ratio (ACR), and protein/creatinine ratio (PCR) in the assessment of proteinuria. Created by Marina de Cos Gomez. Based on: Glassock RJ. Kidney Int. 2016 Nov;90(5):938-940. Rodby RA. Am J Kidney Dis. 2016 Dec;68(6):836-838. Constantiner M, et al. Am J Kidney Dis. 2005 May;45(5):833-41.
| Urine dipstick | 24-hour excretion rate | PCR and ACR | |
|---|---|---|---|
| Basis | Albumin on a buffer causing a change in pH → change in the color of a paper strip. | 24 h collection. Different techniques (chemical, dye binding, turbidimetric). |
Corrected by creatinine excretion (≈1g/d). Different techniques. |
| Pro | Easy and economical. Sensitive to albumin. |
Method of reference. Averages variation of Proteinuria Measures all proteins |
Not influenced by hydration or rate of diuresis. PCR measures all proteins |
| Con | Detection limit 0.25-0.3 g/L. Does not detect non-albumin proteinuria Semiquantitative. Influenced by timing, hydration, and urine pH. |
Prone to over and under the collection. | Influenced by creatinine generation (e.g. sex, BMI, diet, glucocorticoids…). Influenced by the timing of sample |
Usually, albuminuria (measured by ACR) is the preferred method for defining and staging CKD. It is also what is typically followed in patients with diabetic kidney disease and correlated with kidney and cardiovascular outcomes. Proteinuria (assessed by PCR or 24-hour collection) is more commonly used for other glomerular diseases and their trends (rather than one absolute value) should be used when making important therapeutic clinical decisions. Since trends are more useful than absolute values, typically the same method of proteinuria/albuminuria quantification is used in the same patient over time.
Are different degrees of proteinuria seen in different glomerular diseases?
While each glomerular disease itself can present with a varying degree of proteinuria in different patients, there are glomerular diseases that are more likely to have nephrotic-range proteinuria (>3.5g / day) and to present with nephrotic syndrome than others.
For example, patients with minimal change disease, focal segmental glomerulosclerosis (FSGS), membranous nephropathy, and amyloidosis are more likely to develop nephrotic-range proteinuria than patients with post-infectious glomerulonephritis (GN) or ANCA-associated GN. This difference along with the presence or absence of active sediment and other organ involvement can help narrow the differential diagnosis for a patient with proteinuria that is suspected to be glomerular in etiology.
How do you work up isolated microscopic hematuria?
Microscopic hematuria (MH) is defined as ≥3 RBCs/high powered field (hpf) on urine microscopy without visible red color in the urine. Isolated MH is defined as MH without significant proteinuria (<500mg/day) or kidney dysfunction. Positive blood on dipstick does not define MH and urine microscopy is required to confirm the presence of RBCs for diagnosis. The approach to isolated microscopic hematuria is summarized in here:
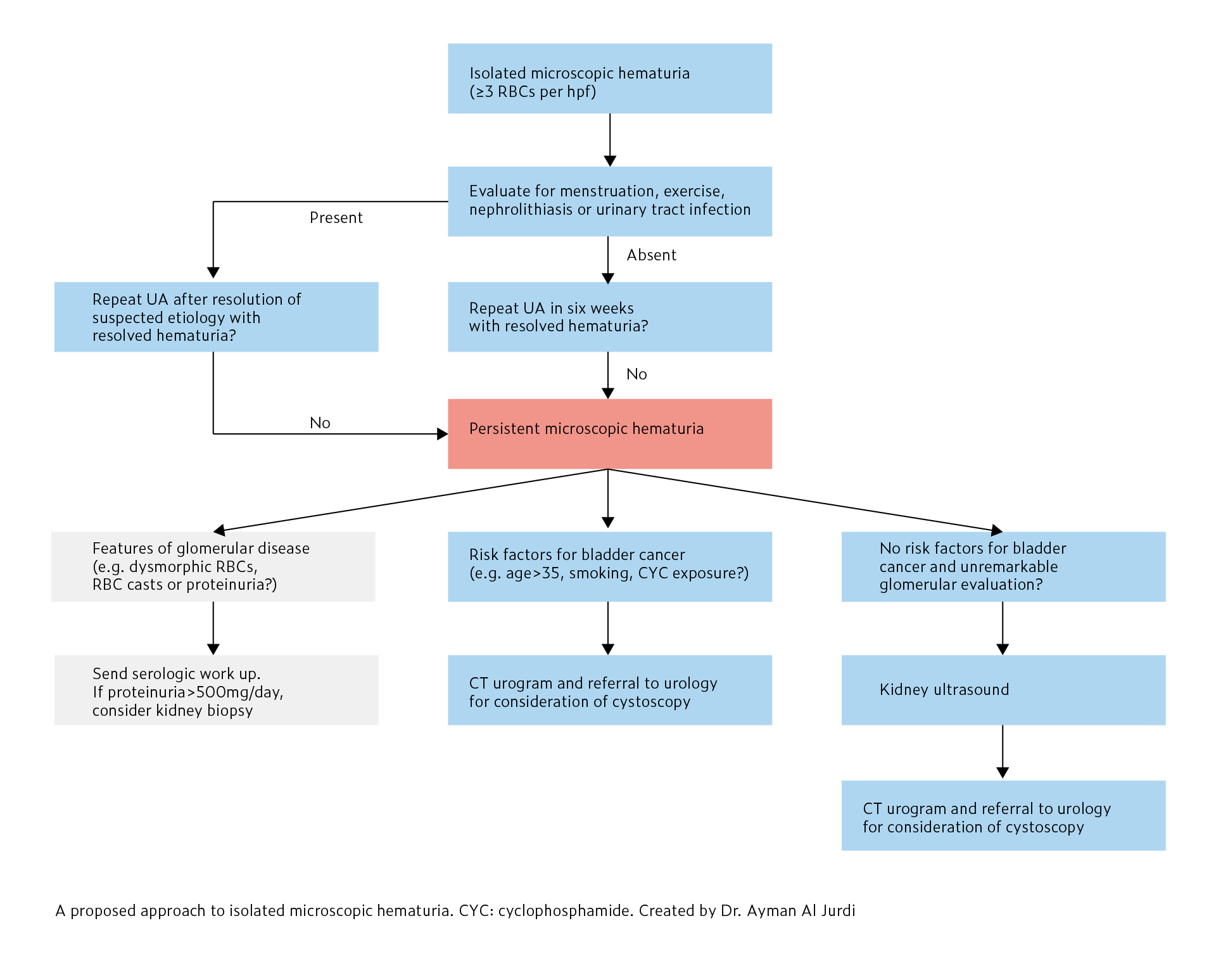
In patients with confirmed MH, the next step is to evaluate for benign etiologies such as menstruation, vigorous exercise, nephrolithiasis, and urinary tract infections. After benign etiologies are evaluated and treated, urine microscopy should be repeated in six weeks to confirm the resolution of MH.
In patients without any of the above benign etiologies or those with persistent hematuria despite treatment of the presumed benign etiology, a primary kidney or malignant etiology should be considered. Evaluation of the urine sediment can be helpful as the presence of acanthocyte-uria or RBC casts are consistent with glomerular hematuria, as would proteinuria or renal insufficiency. In these patients, serologic evaluation and kidney biopsy need to be considered. A kidney biopsy is not typically recommended in isolated MH (without kidney insufficiency and <500mg/day of proteinuria) as it is unlikely to change management.
In patients with risk factors for bladder cancer (e.g. age>35, smoking, occupational exposures, previous cyclophosphamide exposure) or no clear glomerular etiology of MH, a CT urogram and referral to urology is recommended for consideration of cystoscopy to evaluate for malignant etiologies of MH. In young patients (<35 years old) without evidence of glomerular disease or risk factors for bladder cancer, a kidney ultrasound should be performed.
Take home points:
- Proteinuria is defined as urinary protein excretion of greater than 150mg per day.
- The etiologies of proteinuria can be classified into overflow, glomerular, tubular, and post-kidney proteinuria.
- Glomerular proteinuria is predominantly albuminuria (UACR/UPCR usually >40%) and can reach nephrotic-range (>3.5g / day), while tubular proteinuria is predominantly non-albumin proteinuria (UACR/UPCR usually <40%) and is usually <2g/day. Glomerular diseases where the main target is the podocytes (e.g. minimal change disease) tend to result in heavier proteinuria compared to glomerular diseases that do not target podocytes (e.g. ANCA-associated vasculitis).
- Each method of proteinuria quantification has advantages and disadvantages that have to be considered. Albuminuria (usually UACR) is usually used for CKD classification and in patients with diabetic kidney disease, while proteinuria (usually UPCR or 24-hour collection) is used in patients with glomerular diseases.
- The approach to microscopic hematuria includes the exclusion of benign etiologies (e.g. menstruation) followed by a workup targeted towards glomerular etiologies (in the presence of proteinuria, RBC casts, or acanthocytes) and/or post-kidney etiologies (e.g. bladder cancer). Kidney biopsies are usually not performed for isolated microscopic hematuria in the absence of abnormal GFR or significant proteinuria (>500mg/day).

How do you treat this condition with some one who has it that has diabetes and no health insurance