TREATMENT
Treatment of Lupus Nephritis: Practical Insights
Acknowledgment: Based on GlomCon Virtual Fellowship workshop “The Treatment of Lupus Nephritis” by Dr. Joanna Kent and Dr. Desmond Yap, December 10, 2020.
This workshop addressed the following issues in the management of lupus nephritis:
1. Deliverability of cyclophosphamide and MMF induction regimens
2. Utility of repeat kidney biopsy in the management of lupus nephritis
3. Approach to worsening/refractory disease and role of multi-target/novel therapies
1. Deliverability of cyclophosphamide and MMF induction regimens
2. Utility of repeat kidney biopsy in the management of lupus nephritis
3. Approach to worsening/refractory disease and role of multi-target/novel therapies

By Dr. Paolo Nikolai So
Nephrology Fellow
Philippine General Hospital, Philippines
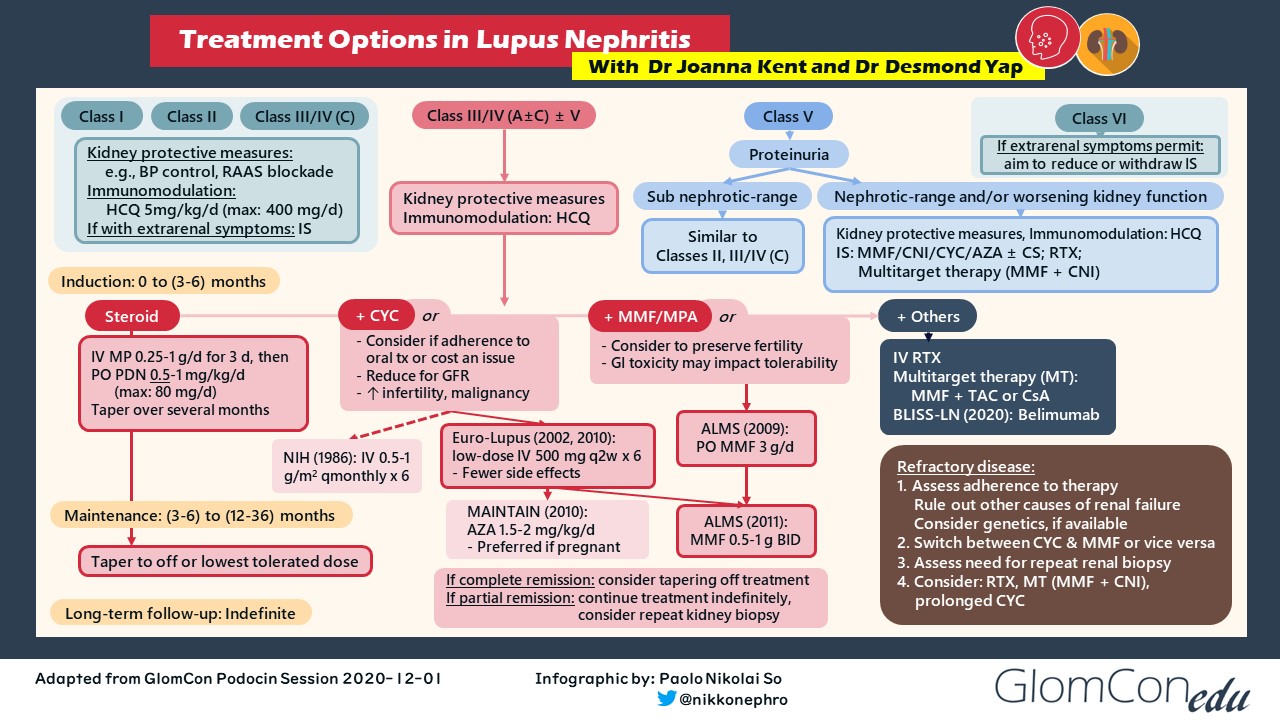
The development of lupus nephritis (LN) in individuals with systemic lupus erythematosus (SLE) is associated with higher rates of mortality. Despite advances in the understanding of its pathobiology, developments in LN treatment remain scarce. The figure below shows a simplified treatment algorithm of lupus nephritis, indicating the landmark trials that served as the basis for such immunosuppressive regimens.
Treatment of individuals with proliferative lupus nephritis (i.e., class III/IV ± V) is vital in lupus management because these patients are at the highest risk of progression necessitating kidney replacement therapy. Well-known standard-of-care (SOC) induction regimens involve high-dose corticosteroids and either cyclophosphamide (CYC) or mycophenolate mofetil (MMF). Intravenous CYC was first popularized by the 1986 NIH study, but it was shown to be associated with an increased risk of premature ovarian failure and malignancy. Hence, lower doses of IV CYC were investigated and were shown to have similar renal outcomes. Mycophenolate mofetil is an equally efficacious induction agent compared with IV CYC, but gastrointestinal toxicity may impact tolerability. In such instances, MMF may be switched to enteric-coated mycophenolate sodium (EC-MPS), which was shown in randomized controlled trials involving kidney transplant recipients to have fewer gastrointestinal symptoms and better patient tolerability. Therapeutic drug monitoring may also be performed with a mycophenolic acid test to better guide drug dosing, but the use of such assay is currently limited to patients taking MMF.
What factors should be considered when choosing a CYC- or MPA-based induction regimen?
IV CYC may be considered if cost is an issue. Increased adherence to treatment may be observed in selected patients with intermittent IV therapy rather than daily oral medication. For patients with severe LN requiring dialysis, there is anecdotally more confidence with the use of CYC in terms of efficacy than MMF. Whereas CYC is dialyzable, MMF is not, making its pharmacokinetics more unpredictable in this patient population. In contrast, mycophenolate mofetil may be more effective than CYC in high-risk populations (e.g., African Americans, Hispanics). Current data are also insufficient for using a reduced-dose IV CYC regimen in the Asian population. Some Asian regions, however, may encounter barriers to access to MMF. Ultimately, the choice of induction therapy is based on an extensive patient-physician discussion on medication risk-benefit profile, health economics and access, and patient preference and adherence.
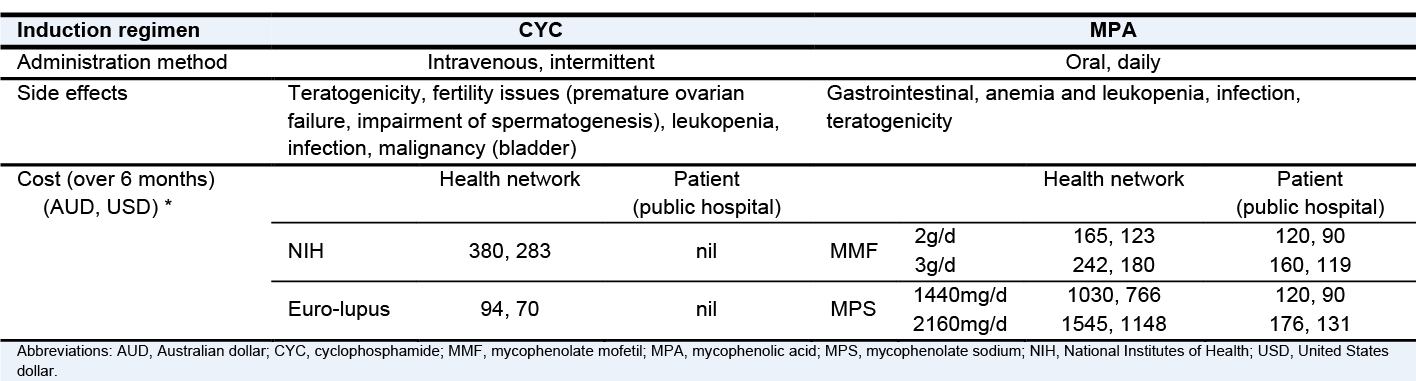
* The cost estimates relate to the Australian health system
Adapted from Dr. Joanna Kent’s GlomCon Virtual Fellowship presentation, 10th December /2020
For patients who have achieved clinical remission after induction therapy, the duration of maintenance treatment ranges from 6 months to more than 2.5 years. The optimal duration of maintenance immunosuppression has not yet been established, as the use of clinical markers (e.g., urinary protein, serum creatinine, complement, anti-dsDNA levels) may not accurately estimate actual histologic remission. Basing maintenance immunosuppression duration on arbitrary timeframes or on clinical parameters may lead to premature discontinuation of maintenance immunosuppression leading to LN flares, or to undue exposure to immunosuppressive medications along with their potential side effects.
Would protocolized kidney biopsies be helpful in guiding the management of maintenance immunosuppression?
In a recent study, biopsy-guided maintenance immunosuppression was shown to be informative in identifying patients whose immunosuppressive therapy can be safely withdrawn. It displayed an exceptionally low rate of LN flare, but head-to-head studies are still underway to rationalize the routine use of such an invasive approach. REBIOLUP (NCT04449991) is an ongoing randomized clinical trial comparing post-induction management of patients with incident biopsy-proven proliferative or membranous LN with or without a per-protocol repeat kidney biopsy in terms of renal outcomes.
Most patients with proliferative LN respond well with standard therapy. A lack of response often reflects incomplete adherence to medications. Repeat kidney biopsy may be considered for patients with worsening disease to assess the histologic burden and to exclude other renal pathology. For rare cases of refractory LN, the following treatment options may be considered:
- switch between MMF and CYC,
- rituximab as an add-on or alternative therapy,
- a combined calcineurin inhibitor (CNI) multitarget therapy,
- prolonged courses of IV CYC, or
- enrollment in clinical trials.
What advances in LN therapy could we expect down the pipeline?
Calcineurin inhibitors are currently being used as an alternative therapy for lupus nephritis, especially in those with a membranous component (i.e., class V ± III/IV). Multitarget therapy with TAC and MMF was shown to be more effective in inducing remission than IV CYC, while TAC combined with corticosteroids has been suggested to have a role as a long-term maintenance agent. Long-term data regarding the efficacy of these regimens with respect to outcomes like renal flare rate and progression to ESKD are awaited. In recent years, low-dose voclosporin as an add-on immunosuppressant for induction showed better clinical response than standard treatment alone based on the AURA-LV (Phase 2) and AURORA (Phase 3) studies. The success of CNIs in LN treatment partly stems from their ability in stabilizing the actin cytoskeleton, thereby causing an additional anti-proteinuric effect on top of their role in immunosuppression.
B cell-depleting agents are also being used in the management of LN. Although rituximab failed to show an increase in renal response rates in the LUNAR study, belimumab added to standard therapy was shown in BLISS-LN to have a better primary efficacy renal response in patients with active LN than standard therapy alone. Obinutuzumab was also shown to have improved renal responses when added to SOC therapy in the NOBILITY trial (Phase 2) (NCT02550652) and is currently being investigated in REGENCY (Phase 3) (NCT04221477).
Key learning points:
- Development in LN treatment is currently evolving.
- Initial choice of standard induction regimen is individualized based on medication risk-benefit profile, health economics and access, and patient preference and compliance.
- Biopsy-guided maintenance immunosuppression shows promise in reducing LN flares.
- Management of refractory LN involves evaluating medication adherence, consideration of a repeat biopsy (to distinguish between ongoing histological activity versus chronic damage), and a change in therapy.
- Emerging novel therapies targeting specific pathways are presently undergoing randomized clinical trials, with positive results emerging.
References:
- Austin HA 3rd, Klippel JH, Balow JE, et al. Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med. 1986;314(10):614-619. doi:10.1056/NEJM198603063141004
- Luo W, Mao P, Zhang L, et al. Assessment of ovarian reserve by serum anti-Müllerian hormone in patients with systemic lupus erythematosus: a meta-analysis. Ann Palliat Med. 2020;9(2):207-215. doi:10.21037/apm.2020.02.11
- Flanc RS, Roberts MA, Strippoli GF, et al. Treatment of diffuse proliferative lupus nephritis: a meta-analysis of randomized controlled trials. Am J Kidney Dis. 2004;43(2):197-208. doi:10.1053/j.ajkd.2003.10.012
- Houssiau FA, Vasconcelos C, D’Cruz D, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002;46(8):2121-2131. doi:10.1002/art.10461
- Houssiau FA, Vasconcelos C, D’Cruz D, et al. The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis. 2010;69(1):61-64. doi:10.1136/ard.2008.102533
- Appel GB, Contreras G, Dooley MA, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20(5):1103-1112. doi:10.1681/ASN.2008101028
- Shehata M, Bhandari S, Venkat-Raman G, et al. Effect of conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium on maximum tolerated dose and gastrointestinal symptoms following kidney transplantation. Transplant International. 2009;22(8):821-830. https://doi.org/10.1111/j.1432-2277.2009.00877.x
- Ortega F, Sánchez-Fructuoso A, Cruzado JM, et al. Gastrointestinal quality of life improvement of renal transplant recipients converted from mycophenolate mofetil to enteric-coated mycophenolate sodium drugs or agents: mycophenolate mofetil and enteric-coated mycophenolate sodium. Transplantation. 2011;92(4):426-432. doi:10.1097/TP.0b013e31822527ca
- Yap DY, Chan TM. Lupus nephritis in Asia: Clinical features and management. Kidney Dis (Basel). 2015;1(2):100-109. doi:10.1159/000430458
- Malvar A, Alberton V, Lococo B, et al. Kidney biopsy-based management of maintenance immunosuppression is safe and may ameliorate flare rate in lupus nephritis. Kidney Int. 2020;97(1):156-162. doi:10.1016/j.kint.2019.07.018
- Yo JH, Barbour TD, Nicholls K. Management of refractory lupus nephritis: challenges and solutions. Open Access Rheumatol. 2019;11:179-188. doi:10.2147/OARRR.S166303
- Rivera F, Mérida E, Illescas ML, et al. Mycophenolate in refractory and relapsing lupus nephritis. Am J Nephrol. 2014;40:105-112. doi:10.1159/000365256
- Weidenbusch M, Römmele C, Schröttle A, et al. Beyond the LUNAR trial. Efficacy of rituximab in refractory lupus nephritis. Nephrol Dial Transplant. 2013;28(1):106-111. doi:10.1093/ndt/gfs285
- Cortés-Hernández J, Torres-Salido MT, Medrano AS, et al. Long-term outcomes -mycophenolate mofetil treatment for lupus nephritis with addition of tacrolimus for resistant cases. Nephrol Dial Transplant. 2010;25(12):3939-3948. doi:10.1093/ndt/gfq322
- Choi CB, Won S, Bae SC. Outcomes of multitarget therapy using mycophenolate mofetil and tacrolimus for refractory or relapsing lupus nephritis. Lupus. 2018;27(6):1007-1011. doi:10.1177/0961203318758505
- Mok CC, Ying KY, Ng WL, et al. Long-term outcome of diffuse proliferative lupus glomerulonephritis treated with cyclophosphamide. Am J Med. 2006;119(4):355.e25-355.e3.55E33. doi:10.1016/j.amjmed.2005.08.045
- Liu Z, Zhang H, Liu Z, et al. Multitarget therapy for induction treatment of lupus nephritis: a randomized trial. Ann Intern Med. 2015;162(1):18-26. doi:10.7326/M14-1030
- Yap DY, Ma MK, Mok MM, et al. Long-term data on tacrolimus treatment in lupus nephritis. Rheumatology (Oxford). 2014;53(12):2232-2237. doi:10.1093/rheumatology/keu265
- Rovin BH, Solomons N, Pendergraft WF 3rd, et al. A randomized, controlled double-blind study comparing the efficacy and safety of dose-ranging voclosporin with placebo in achieving remission in patients with active lupus nephritis. Kidney Int. 2019;95(1):219-231. doi:10.1016/j.kint.2018.08.025.
- Rovin BH, Parikh SV, Huizinga RB, et al. Management of lupus nephritis (LN) with voclosporin: An update from a pooled analysis of 534 patients. Presented at: Kidney Week 2020 Reimagined virtual conference, October 19 to 25. Poster PO1917.
- Rovin BH, Furie R, Latinis K, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012;64(4):1215-26. doi:10.1002/art.34359.
- Furie R, Rovin BH, Houssiau F, et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med. 2020;383(12):1117-1128. doi:10.1056/NEJMoa2001180.
- Furie R, Aroca G, Alvarez A, et al. A phase II randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of obinutuzumab or placebo in combination with mycophenolate mofetil in patients with active class III or IV lupus nephritis. Arthritis Rheumatol. 2019;71: [Abstract].
