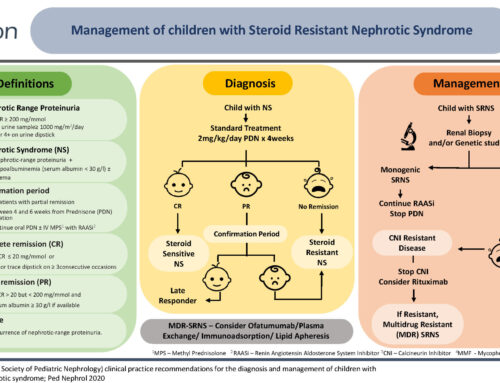
CLINICAL TRIALS
Pediatric GN Trials
In this fourth episode of the Clinical Trial Conference Series (a joint venture between NephCure and GlomCon), Dr. Debbie Gipson chaired a discussion of pediatric trials in glomerular diseases. Background epidemiology, practical experiences, and regulatory considerations were addressed by speakers Drs Keisha Gibson, Carla Nester, Jula Inrig, and research nurse Suzanne Vento. Our Moderator’s Notes are derived from this live presentation


By Dr. Kate Robson
Background:
Childhood Nephrotic Syndrome
- onset typically 2-7yo
- affects boys > girls 2:1
- BP and kidney function typically normal,
- BUT up to 15-20% have haematuria/abnormal kidney function
- Is a syndrome, not a disease diagnosis
- Underlying diseases include:
- Minimal Change Disease (MCD)
- Focal Segmental Glomerulosclerosis (FSGS)
- Other (IgA nephropathy/vasculitis, C3 glomerulopathy, lupus nephritis)
Minimal Change Disease (MCD)
- Affects 1-3 per 100,000 children in USA
- Usually responsive to treatment, but >70% have relapses
- Relapses usually become milder and less frequent with time, and often stop in adolescence
- Children are more likely to experience relapses into adulthood if disease onset at <6yo
Focal Segmental Glomerulosclerosis (FSGS)
- Represents 15-20% of childhood nephrotic syndrome
- Onset usually older than MCD (50% are >6yo at onset)
- More common in African-American & Hispanic populations
- Low rate of response to currently available therapies
- End-stage kidney disease develops in >25-50%
C3 glomerulopathy
- Characterized by complement dysregulation
- In most patients, disease is driven by acquired factors: autoantibodies targeting complement proteins and complexes. Variants in complement-related genes are less frequently observed.
- Diagnosis is made on kidney biopsy (no diagnostic biomarkers)
- Post-infectious glomerulonephritis cannot be differentiated from C3 glomerulopathy by biopsy alone: this can confound early diagnosis/treatment.
- 50% approach end-stage kidney disease in 10 years.
- After transplantation, clinical recurrence is seen in 50% and histological recurrence in 90%.
- Clinical trials currently in progress with agents targeting the alternative pathway of complement, including inhibitors of MASP, Factor D, Factor B and C3, and the C5a receptor blocker, avacopan. Of these, only the avacopan study is recruiting children (from age 12yo) https://clinicaltrials.gov/ct2/show/NCT03301467
Practical Considerations in Pediatric GN Clinical Trials
- Limited trials in children can mean:
- children do not receive potentially beneficial medications because these agents have not been approved for pediatric use
- children receive medications based on evidence from adult studies and limited anecdotal pediatric clinical experience (off-label use)
- Practical issues in administering clinical trials:
- Is the protocol feasible?
- Do we have enough eligible patients?
- Are there any competing studies?
- Managing referrals from other centres (patient and family unfamiliar with treating team, travel and accommodation logistics)
- Coordinating visit schedules with school/family commitments
- Education about study procedures (e.g. accurate urine specimen collection)
- Identifying active clinical trials:
- clinicaltrials.gov – U.S. National Library of Medicine database of clinical studies worldwide
- kidneyhealthgateway.com – user-friendly portal created by NephCure to help patients with kidney disease identify clinical trials and connect directly with research teams. A physician portal is in development.
Regulatory Considerations in Pediatric GN Clinical Trials
- Regulations in USA and Europe require that sponsors developing a new medication (including new formulation, new route of administration, new indication) submit a plan for investigation in the pediatric population
- Waiver or deferral of pediatric studies is common (e.g. until after evaluation of adult safety data)
- ‘Orphan’ indications (conditions affecting <200,000 individuals – e.g. pediatric GN diseases) are excluded from the mandate
- Companies are incentivized to conduct pediatric studies by the provision of a period of market exclusivity
- A comprehensive pediatric plan established early in drug development not only benefits pediatric patients but assures no delay in market entry
Selected Resources:
https://www.ncbi.nlm.nih.gov/pubmed/26656320
https://www.ncbi.nlm.nih.gov/pubmed/30692664
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6224619/
kidneyhealthgateway.com
clinicaltrials.gov