CLINICAL TRIALS
Clinical Trial Series: IgA Nephropathy
In this session of Clinical Trial Series (a joint venture between NephCure and GlomCon), Drs. Dana Rizk, Heather Reich, Jonathan Barratt, and Richard Lafayette provided a brief review of our current knowledge about IgA nephropathy, new therapeutic targets, and highlight two of the many ongoing trials in IgA nephropathy. Our Moderator’s Notes are derived from this live presentation.


By Dr. Ali Poyan Mehr
Key points:
Background:
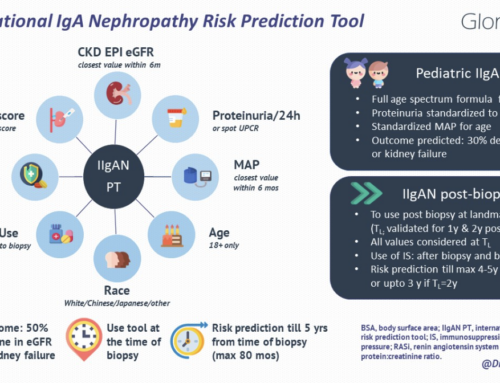
- IgA Nephropathy is worldwide the most common primary glomerulonephritis and a frequent cause of ESRD.
- Several factors are associated with risk for disease progression and ESRD, including persistent proteinuria despite RAAS blockade.
- Prediction tools have been created based on these risk factors.
- Renin-angiotensin-aldosterone system inhibitors are the main ingredient of conservative, non-immunosuppressive therapy.
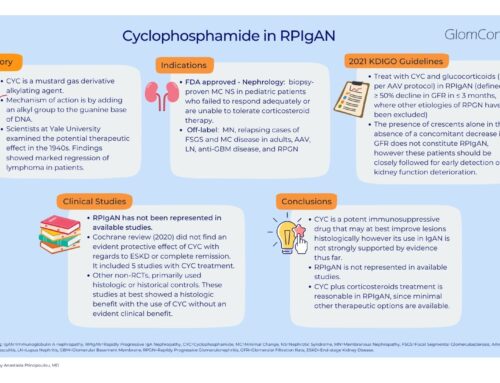
- Steroids have been studies the most among the immunosuppressive therapies, and STOP-IgAN Trial and the TESTING Trial were briefly reviewed. Bottom line: Certain subgroups of patients seem to benefit from steroid therapy. However, more studies are needed to guide us about the risk-benefit and help identify those patients who are most likely to benefit from therapy in the long term.
Knowledge Gaps:
- How to treat IgA nephropathy vs. HSP, vs. IgA vasculitis + nephritis vs. crescentic IgA, etc.?
- Need for further identification of targetable disease pathways.
- Need for validated surrogate markers for clinically meaningful outcomes.
- Research guided patient selection for clinical trials (clinical, pathological, and ethnic differences).
- Lack of disease-specific biomarkers for assessment of disease activity and therapeutic response.
Current Understanding of Targets and Pathways:
- Mucosa-associated lymphoid tissue (MALT), as the mainline of defense against gut microbiota: This largest source of IgA secretion in the human body is also considered to be the main source of the IgA immunoglobulin in IgA nephropathy.
- B-cell activation (through T-cell dependent and T-cell independent mechanisms) is necessary for IgA production and secretion [note that while B-cell activation is essential for IgA secretion, rituximab, an anti-B-cell antibody, appears to be not efficacious in the treatment of IgA nephropathy]
- Inappropriate galactosylation of the polymeric IgA (galactose deficient) stimulates auto-antibody formation (IgGs) which together with the under galactosylated IgA form immunocomplexes, activate complement, and lead to renal injury.
- Genetic predisposition and likely the gut microbiome are important factors in disease development and progression.
- B-cell activating cytokines are a promising therapeutic target, and some are under current investigation (examples reviewed during the session).
- As IgA is also secreted by plasma cells, anti-plasma cell therapy is potentially a promising therapeutic option.
- Targeting complement pathway for the treatment of IgA nephropathy is also under investigation, given a subset of patient display significant complement activation and tissue deposition [note: the IgA-IgG immunocomplexes have the capability of binding and activating complement system].
Due to this strong link of the mucosal immune system and IgA nephropathy, many patients often wonder about the influence of diet (e.g., gluten-free) in the development, progression, and mitigation of IgA nephropathy. Due to the lack of evidence for a specific diet being causative or protective, no specific recommendation can be made, other than healthy, low sodium, and a balanced diet void of heavily processed ingredients.
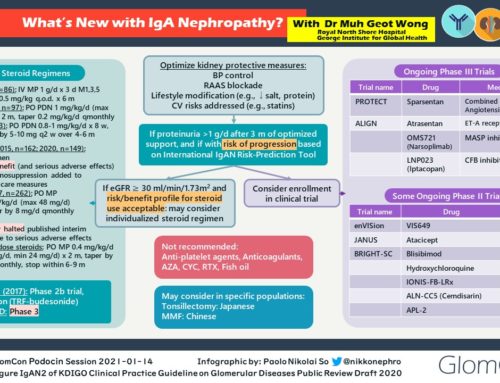
Nefigard Study
(https://clinicaltrials.gov/ct2/show/NCT03643965?term=NCT03643965&rank=1)
- Rationale: Strong link between mucosal immune-activation (mucosa-associated lymphoid tissue) and IgA nephropathy.
- Glucocorticoids act as strong inhibitors of the immune system, including inhibition of immunoglobulin production.
- Targeted release of glucocorticoids (budesonide) at the level of the mucosa may be more efficacious in suppressing the production of under galactosylated IgA, while at the same time limit systemic adverse effects often observed in glucocorticoid therapy.
- Study design: Phase 3, randomized, double-blind, placebo-controlled.
- Patients must be on max tolerated RAAS blockade and still have a urine protein to creatinine ratio ≥1 g/24hr.
- Primary Outcome: 1) Change in proteinuria at nine months compared to baseline: UPCR (based on 24-hour urine collections) at nine months following the first dose of study drug compared to baseline, and 2) Events based on eGFR measure compared to baseline calculated using the CKD-EPI formula
- Secondary Outcome: The incidence of treatment-emergent adverse events, and renal function measured as eGFR using the CKD-EPI formula
ARTEMIS_IGAN Study
(https://clinicaltrials.gov/ct2/show/NCT03608033?term=NCT03608033&rank=1)
- Rationale: Complement system is implicated in the pathogenesis of IgA nephropathy (above).
- OMS721 (Narsoplimab), a monoclonal antibody is a human monoclonal antibody targeting mannan-binding lectin-associated serine protease-2 (MASP-2) and the effector enzyme of the lectin pathway of the complement system.
- Study design: Phase 3, double-blind, randomized, placebo-controlled.
- Patients must have a urine protein to creatinine ratio of >1 g/g at the time of screening (mean of two measurements).
- Primary outcome: Change from baseline in 24-hour urine protein excretion in g/day at 24 weeks.
- Secondary outcome: 1) Treatment-related Adverse Events throughout 168 Weeks, 2) change from baseline in renal function as determined by the rate of change in eGFR up to 144 weeks from beginning of treatment, 3) change from baseline in 24-hour urine protein excretion in g/day at 36 weeks from beginning of treatment in the subset of patients with baseline high proteinuria (defined as 24-hour urine protein excretion ≥ 2 g/day), 4) Time-averaged change in urine protein/creatinine ratio through 36 weeks.
A panel discussion about identifying patient who would benefit most from clinical trial participation, barriers to trial participation, and other questions were addressed throughout the session.
References:
- Rodrigues JC, Haas M, Reich HN. IgA Nephropathy. Clin J Am Soc Nephrol. 2017 Apr 3;12(4):677-686. https://www.ncbi.nlm.nih.gov/pubmed/28159829
- Rizk DV, Maillard N, Julian BA, Knoppova B, Green TJ, Novak J, Wyatt RJ. The Emerging Role of Complement Proteins as a Target for Therapy of IgA Nephropathy. Front Immunol. 2019 Mar 19;10:504. https://www.ncbi.nlm.nih.gov/pubmed/30941137