PERSPECTIVE
Shortcomings of the current GFR estimating equations
When we estimate the glomerular filtration rate of a patient, we currently use two different formulas, one for African American patients and one for non-African Americans (everyone else). Using ethnicity and ancestry to tailor medicine may have been well-intentioned, but it is like a software that is full of bugs, and for which we do not have a patch yet. Here, a brief review of the currently most frequently used creatinine clearance and GFR estimating formula (Cockcroft and Gault, MDRD, and CKD-EPI) and their limitations in daily practice.


By Dr. Ali Poyan Mehr
Ethnicity and Ancestry:
Humans are ethnically diverse, and our “race-based” categorization into distinct groups is not based on consistent genetic or biological characteristics, but solely based on how we perceive others to be “different.” The U.S. Census Bureau has institutionalized the notion of group differences in the U.S. by asking individuals to identify as distinct ethnic groups. Among these categories is listed “African-American.”
Although designating someone as “African America” is seemingly a simple task (anyone of African descent or with African ancestry), the reality is complicated. Is someone “African American” if all ancestors are from Africa, or does it suffice if only one person in the family tree originated from Africa? Or perhaps 25% or 50% of paternal and maternal ancestors? Maybe it is only the maternal lineage that matters? How does someone who self-identifies as “African American” makes these distinctions, and how does it compare to when others, such as caregivers or scientists, judge a person to be “African American”?
Some additional background was provided in a series of Tweets by Dr. Gearoid M. McMahon, a Nephrologist at the Brigham and Women’s:
A follow on from recent discussions about race and eGFR.
— Gearóid McMahon (@gearoidmm) July 16, 2020
In 1930, the US census bureau removed "mulatto" as a racial descriptor formally incorporating the "one-drop rule" that created a false dichotomy between black and white races https://t.co/WPecZlrcEW 1/
This was an effort to ignore the fact that there had been considerable mixing between people of black and white ancestry and was yet another effort to codify segregation. 2/
— Gearóid McMahon (@gearoidmm) July 16, 2020
The idea that the color of your skin alone can be used to categorize your risk of disease is a medical remnant of these old ideas. As nephrologists we should have learned from the APOL1 story that race is a poor surrogate for the true reasons for differences in outcomes. 3/
— Gearóid McMahon (@gearoidmm) July 16, 2020
We should take this moment as an opportunity to understand the real reasons for disparities in outcomes between people of different ancestry and develop new tools that incorporate these factors.
— Gearóid McMahon (@gearoidmm) July 16, 2020
Moreover, why are we even making such a distinction within the society in general?
Why are we making such a distinction in medicine more specifically?
Is it to provide tailored medical care because different ethnicities require different treatment options?
What is the scientific evidence supporting this categorization?
When and how has it proven to improves outcomes?
Moreover, if we conclude that labeling “African ancestry” based on the color of skin can help patients receive tailored screening and medical therapies, how do we differentiate between West African and East African ancestry? After all, we know that there is considerable genetic diversity among the different African ethnicities than is between an African sub-population and the white Europeans?
APOL1 and FSGS
Perhaps, focal segmental glomerulosclerosis (FSGS) due to APOL1 risk genotype can serve as an example of how ancestry based medical practice can help or harm a given patient population. Studies have shown that the disease-causing variants of this gene are almost exclusively found in patients with African ancestry. In these patients, the risk of developing FSGS is substantially higher than in other ethnicities, and likely accounting for the disproportionately higher number of African American patients on dialysis in the U.S.
The knowledge that a person with African ancestry is at high risk for FSGS and progressive kidney disease may help implement early screening and additional supportive care for patients who self-identify as African American. Early recognition of the disease, after all, may help alleviate secondary complications, delay progression, or allow enrollment into clinical trials for APOL1 associated kidney disease. However, it matters whether the patient is of West African, East African, North African, or South African descent. Africa is not one country, but a continent that is ethnically and genetically more diverse within than humans are elsewhere. The APOL1 risk variant that is associated with kidney disease has a high prevalence in West Africa but is near zero in some EAST, South, and North African countries and ethnicities.
Moreover, it can also become easy to ascribe all proteinuria to the APOL1 gene risk variant in patients who are categorized as being African American (self-identified or presumed by the healthcare team). Diagnostic anchoring based on the ethnic background can lead to missed diagnosis due to improper workup and consideration of other etiologies. We have seen and do continue to see this abound in African American patients, who are diagnosed with end-stage renal disease due to “hypertensive nephrosclerosis.” Too often, this diagnosis is given to patients without a thorough workup based on the assumption that African American patients have chronic hypertension, which has led to kidney disease. At the same time, and all along, it has been the underlying kidney disease, which has led to hypertension.
Ethnic Diversity
Based on the above, a simple categorization relying on remote ancestry from a continent is imprecise and may not accurately predict risk and disease.
Further complicating is that ethnic origins do not remain frozen in time, and lineages change over generations. How does a patient self-identify whose father is Nigerian and mother Chinese? As African American or Asian? Or what about a dark-skinned Latino? How does the self-identification compare to what care provider may assume when applying “race” based formulae and decision trees?
At what shade of skin color do we consider someone white, Latino, or Black?
Although we could endlessly go into the imprecision and inaccuracies of how individuals, clinicians, society, and bureaucrats designate a given individual as African American, at the end of the day, it comes mostly down to skin color. Therefore, any algorithm that aims to distinguish between “African American” and “non-African American,” but does not take into consideration the ethnic origin, the composition of ancestry, and the diversity of heritage, is an algorithm that merely cuts through the line of how we visually and socially perceive someone as different. This may be “Black” versus “non-Black,” but might as well be left-handed versus right-handed, catholic versus protestants, etc.
The Common Formulas
The Cockcroft and Gault formula. This formula is old and outdated, but still widely referenced in pharmacy literature and for drug dosing purposes. Its background and limitations can be a helpful guide toward understanding skin-color based discrimination of estimating GFR formulae:
- Created to estimate creatinine clearance (not GFR)
- Based on 249 men (assumed to be likely all white soldiers/veterans) in 1976.
- Out of more than 500 men who had 24-hour creatinine measurements available, the 249 subjects were selected (questionable subject selection method).
- The formula includes serum creatinine, age, weight, and sex.
- Here is how the “correction” factor for women was introduced:

- Cockcroft and Gault also confirmed several prior reports and anecdotal experiences that suggested muscle mass influence serum creatine, and therefore affect estimated clearances based on serum creatinine values

- The authors caution about the limitations of this equation and its use in patients with low muscle mass, liver disease, or poor nutritional status. Nevertheless, it has not’ prevented anyone from using it routinely to dose adjust medication in patients with liver disease, in critical care, or other conditions, in which there is reasonable chance to suspect the estimates to be way off and not applicable.
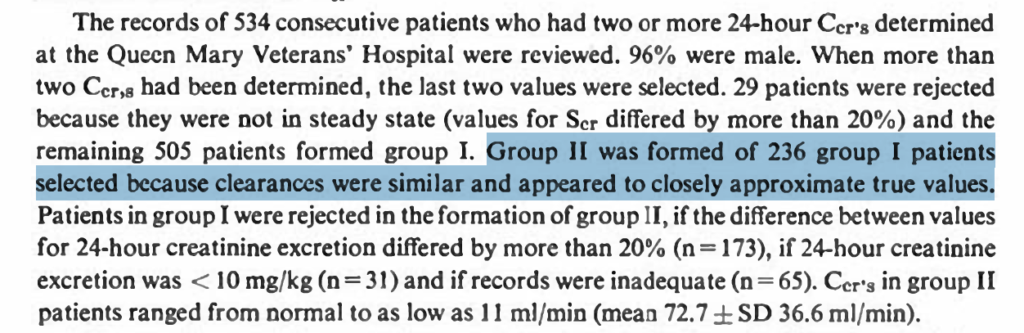
- In addition to serum creatinine, weight, sex, and age are included as correction factors. Age, because with older age, there is less creatinine clearance for the same creatinine value, meaning a serum creatinine of 1 mg/dL in a 70-year-old man corresponds to a lower creatinine clearance than a serum creatinine of 1 mg/dL in a 20-year-old man.
- The difference between the estimated creatinine clearance and the measured creatinine clearance was up to 35% (!) in 95% of patients (and that despite a homogenous base study population, and an equally homogenous validation cohort). That means if creatinine clearance was estimated to be 60 ml/min, then the measured creatinine clearance could be anywhere between 39 to 81 ml/min. This 35% spread is pretty much constant across the entire range of serum creatinine values (from low to high). Though the absolute number of error becomes smaller with higher creatine values and lower clearances (e.g., 35% of 100ml/min is +/-35 ml/min = a range of 70 ml/min, while 35% of 10 ml/min is 3.5 ml/min = a range of 7 ml/min
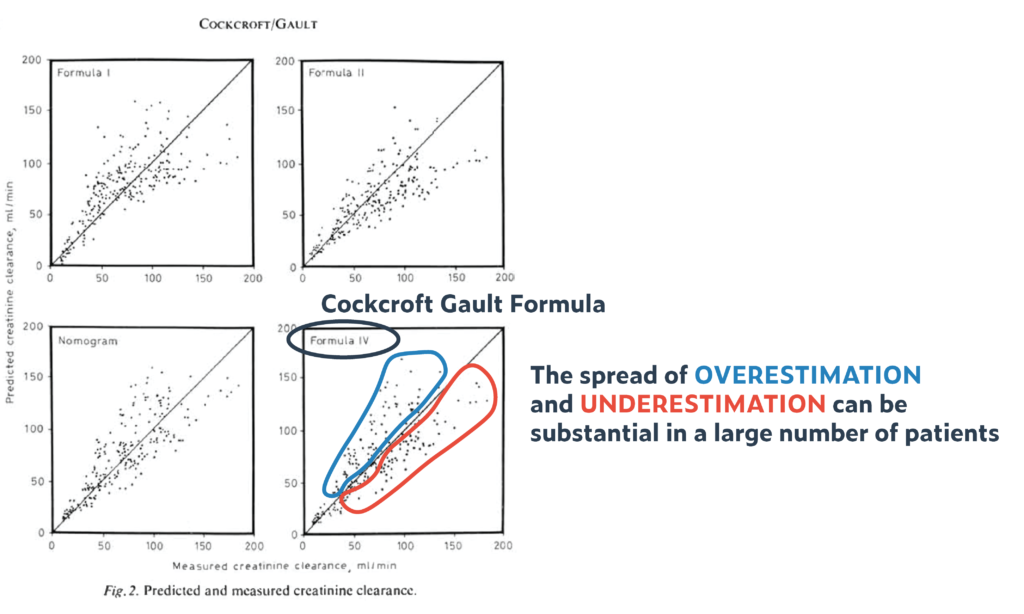
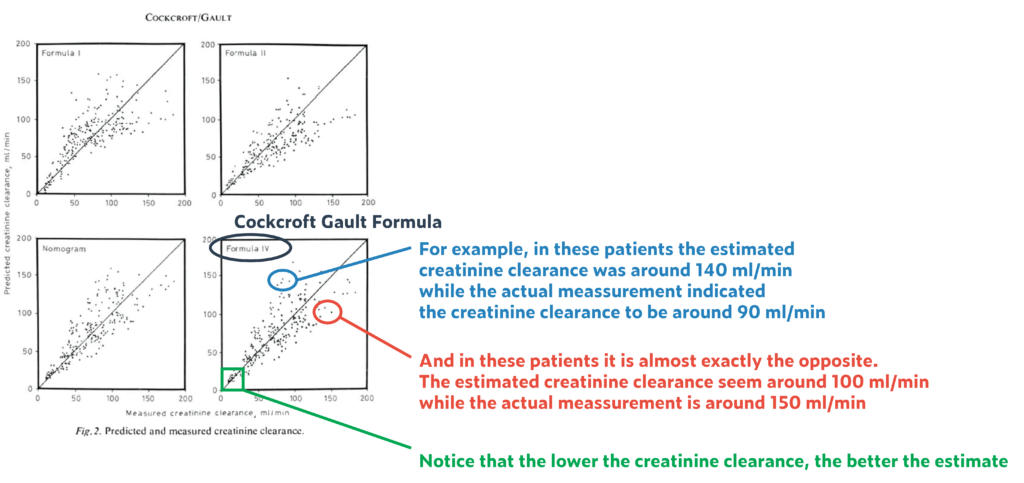
- It was not from direct scientific measurements that Cockcroft and Gault introduced a correction factor of 15% for women, but based on observations that, on average, for the same creatinine, a woman’s creatinine clearance was between 10-20% lower. The authors decided to go with 15% as a middle ground (using 0.85 as correction factor). Further, there was no confirmation (validation) of this correction factor in women.
- Finally, it was not lost to the authors that creatinine clearance is a poor marker of glomerular filtration rate. Adding the impression of deducing GFR from creatinine clearance to the imprecision of driving creatinine clearance from serum creatinine, the results we get can be as good as throwing darts at a moving target, blindfolded.

- Nevertheless, this has not prevented generations of physicians using the Cockcroft and Gault based estimate of creatinine clearance interchangeable as a GFR estimate. Frequently perpetuated and further ingrained by our nephrology medical literature.
- So, why does Cockcroft and Gault’s formula still matter in 2020?
Some argue that if we do away with skin-color-based eGFR discrimination, then we may end up underdosing antibiotics in patients, in whom we now apply the lower eGFR value. Alternatively, if we provide an eGFR range, we may end up overdosing some medication if we erroneously assume the higher end of the eGFR range.
Let’s use enoxaparin and rivaroxaban, two modern-day drugs with substantial adverse risk if overdosed in patients with kidney disease, as an example.
For enoxaparin the package-insert states:

For rivaroxaban the package-insert states:
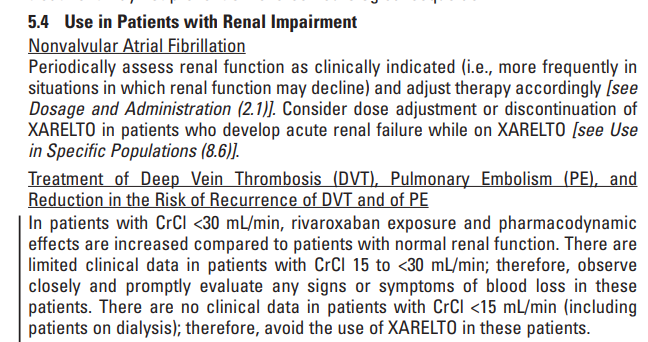
Please pay particular attention to the fact that the manufacturer’s recommendation for dose adjustment and discontinuation is based on “creatinine clearance.”
Note the following:
- Creatinine clearance referenced in this package insert refers to the one estimated by the Cockcroft Gault formula
- As noted, the Cockcroft Gault formula was studied in 249 white men. Women were excluded
- A creatinine clearance that is estimated based on serum creatinine to be 32 ml/min is at best in 95% of cases somewhere between 24 ml/min to 43 ml/min
- Estimated creatinine clearance is NOT estimated GFR. Further, their difference can be 25%-100% apart (see above).
- In 2020 it is safe to assume that 100% of all CLIA certified laboratories and health care organizations do not routinely report creatinine clearance, but creatinine-based estimated GFR (most use MDRD, some use CKD-EPI)
- Nephrologists do not routinely use creatinine clearance for dose adjustment but use the reported eGFR in the medical record. This, however, is not as recommended by the overwhelming majority of package inserts.
- Even pharmacists who review prescriptions for potential renal dose adjustment do so based on eGFR in the medical record, instead of calculating estimated creatinine clearance.
It does not help that scholars and professional societies provide ambiguous and confusing guidance on which one to use and how to use it.
For example, Botev. et al. suggest using eGFR instead of creatinine clearance for drug dosing.

The National Kidney Foundation recommends: (FAQs about GFR Estimates, NKF, accessed 7/5/2020 from https://www.kidney.org/sites/default/files/docs/12-10-4004_abe_faqs_aboutgfrrev1b_singleb.pdf)
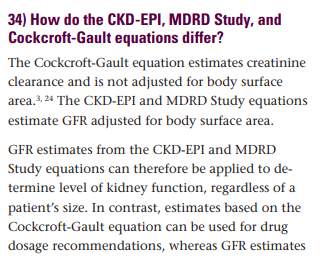
It is this lack of clear distinction between creatinine clearance and eGFR that leads many to use them interchangeably. While in many cases this may not matter as they are in the same “ballpark,” in many cases, they can be vastly different. An additional error is introduced by the lack of awareness that creatinine clearance already accounts for the weight (no adjustment needed), while eGFR (MDRD or CKD-EPI) does not. Probably the body surface area (BSA) should be calculated and accounted for using ideal/lean body weight in patients with large muscle mass (GFR underestimated) or severely obese/anasarca (GFR overestimated), but we do not have the science to guide us here. We will not go into the limitations of indexing for BSA, or how using different formulas for estimating BSA can contribute to substantial differences in BSA adjusted eGFRs. (for more on this check out https://pubmed.ncbi.nlm.nih.gov/17913737/)
Here some case illustration on how different formula may lead to different clinical decisions:
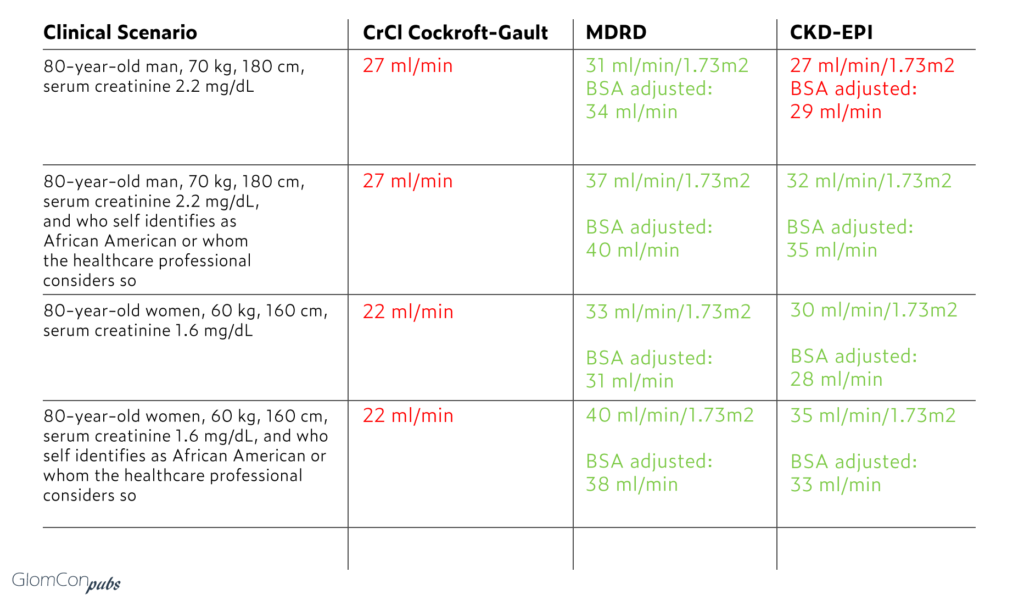
Taking enoxaparin as an example, one can recreate a scenario where the same patient may take enoxaparin without adjustment, with dose adjustment, or not at all, depending on which formula is applied.
In the above example, notice the substantial difference between Cockcroft Gault based creatinine clearance and MDRD or CKD-EPI in patients who are subject to skin color-based adjustment factor. Further, while for the Cockcroft Gault, there is no adjustment to BSA necessary (weight in the formula aims to account for mass differences) the recommendation is to use ideal body weight or the lean body weight in obese patients or patients with volume overload (ascites/edema). However, once again, it is likely that only a minority of clinicians practice that.
While according to the package insert, all four patients above should be receiving a dose-adjusted regimen, in the overwhelming majority of cases, this may not be the case, because:
- The MDRD predicts an eGFR > 30 in all patients
- Clinicians use MDRD reported eGFR interchangeable as creatinine clearance.
- CKD-EPI predicts eGFR> 30 in all but one patient
Based on the imprecision of the estimates, it would be more truthful to report ranges such as a 95% confidence interval (that is the range where 95% of measurements would fall within). In the examples above, for the 80-year-old woman with a creatinine of 1.6 mg/dL, the range where most (95%) of creatinine clearances would fall between 14 ml/min to 30 ml/min. For many medications, this interval can span across several dose adjustment categories: For some patients, that could mean absolute contraindicated (<15 ml/min), and for others, even a dose adjustment would not be required (>=30 ml/min).
As a side note: Remdesivir label advises to use the Cockcroft Gault formula for assessing the kidney function, and alerts of contraindication in patients with a creatinine clearance < 30 ml/min. It is safe to assume that no U.S. medical center follows this recommendation. In the example above, none of the patients should be getting remdesivir according to the label, while in reality, they likely would receive it.
In sum, drug dose adjustment based on eGFR is complicated and fraught with errors. While others have reported on the potential dangers of using Cockcroft Gault and MDRD formula interchangeably for drug dosing ( doi:10.1093/ndt/gfm289 ) our understanding remains limited and the topic poorly studied.
Further, the background scientific information to truly guide dose adjustment in various subpopulations is entirely lacking. Therefore, the elimination of dichotomous eGFR reporting has no bearing on drug dose recommendations that are based on Cockcroft Gault.
Do we risk withholding beneficial medications from patients if we underestimate GFR?
Some proponents of skin-color-based discrimination note that not applying the adjustment factor leads to an underestimate in GFR in some patients who indeed have a higher GFR at a given creatinine value.
The hypothesis is that this underestimation will lead clinicians to withhold essential medications such as metformin or SGLT2 inhibitors, both of which improve the outcome of patients with diabetes and diabetes-related complications, including kidney disease.
This concern, as laudable as it is, does not reflect the realities of day to day practice.
Intra-individual fluctuations in serum creatinine routinely lead to eGFR changes that can be wider in range than the differences observed between the standard formula and the skin-color-based adjustment factor.
In the examples below, that were chosen to reflect the 30 ml/min/1.73m2 dose-adjustment-threshold, the differences in GFR estimates between using skin-color-based discrimination versus not are between 4 to 6 ml/min/1.73m2.
How much longer is a 50-year-old diabetic patient with an eGFR of ~35 ml/min/1.73m2 likely to benefit from an SGLT2 inhibitor before she or he reaches an eGFR of 30 and needs to discontinue the medication?
The CREDENCE trial did show that canagliflozin improves the overall rate of renal decline throughout the study from 4.7 ml/min/1.73m2 to 3.2 ml/min/1.73m2. (The difference in the rate of decline and the benefits seen in the study population (baseline eGFR of ~ 56 ml/min) is higher if not accounting for the initial drop in GFR with the start of the treatment, but likely lower in patients with an eGFR < 35 ml/min/1.73m2).
Here a few examples to illustrate the range of changes in eGFR around the 30 ml/min mark with and without skin-color-based adjustment factor:
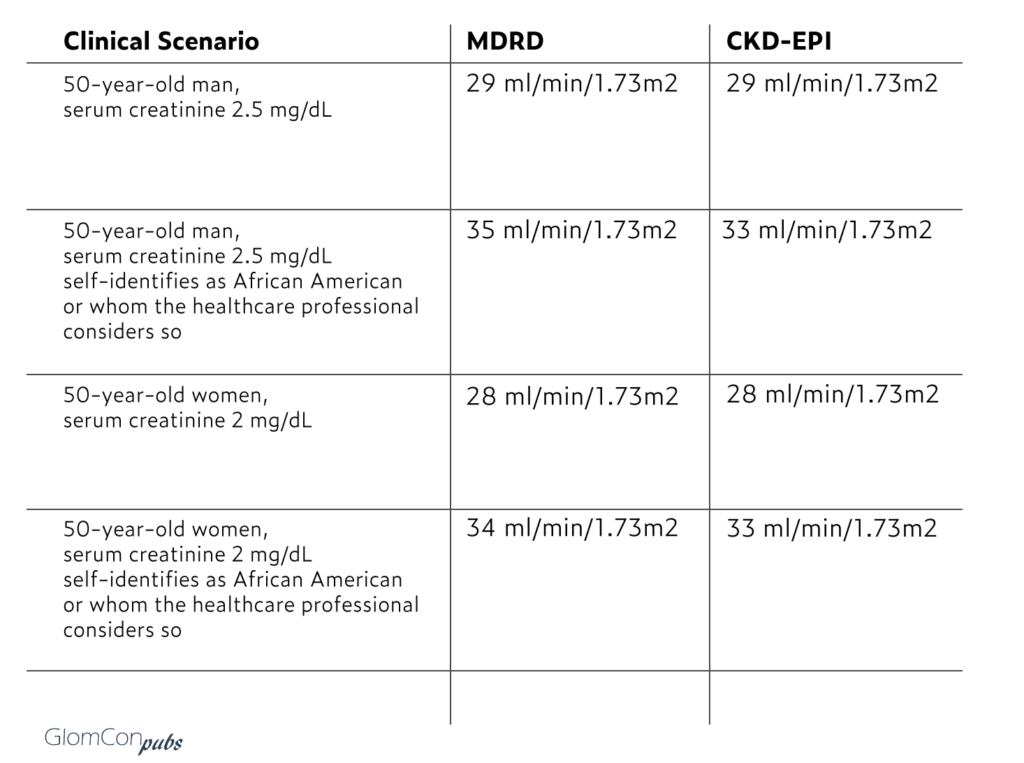
Roughly speaking, a patient with an eGFR of ~35 ml/min/1.73m2 may be able to take canagliflozin for ~ one to two years before reaching an eGFR of 30 ml/min/1.73m2 and having to discontinue due to manufactures recommendation. Furthermore, this even does not account for the possibility that once these critical thresholds are reached, the nephrologists could obtain a 24-hour creatinine clearance measure or use cystatin C for as a less biased (but equally imprecise) eGFR estimate.
Does the benefit of taking metformin of SGLT2 inhibitors for a few months to a year (or perhaps two) longer justify delaying the recognition of kidney disease in specific populations? Does it justify postponing the placement of certain communities on the transplant list?
Would a nephrologist be willing to delay her or his placement on the transplant list by one year or two years for the benefit of taking metformin and SGLT2 inhibitors for a year or two longer?
In sum, the concern of undertreating Black patients with metformin and SGLT2 inhibitors for few months to a couple of years fails to acknowledge that the attempt to provide this benefit comes at a substantial cost: The failure of early detection of kidney disease and timely referral for transplant evaluation in Black patients.
MDRD and CKD-EPI formula:
MDRD was introduced in 1999 to estimate the glomerular filtration rate (not creatinine clearance) based on serum creatinine. They used actual GFR measurements (using measured iothalamate clearance) from 1628 patients, 12% of which were “African American.” Hispanics and Asians made up ~5% and were lumped together with whites.
It is notable that “Ethnicity was assigned by study personnel, without explicit criteria, probably by examination of skin color” https://doi.org/10.7326/0003-4819-145-4-200608150-00004. Therefore, strictly speaking, and scientifically more accurate would be to refer to an algorithm that aims to distinguish between these two cohorts as a skin-color-based adjustment factor.
Bias, Precision, and Accuracy.
To be better informed about the utility of the MDRD and other GFR estimating equations, the concept of bias, precision, and accuracy is worth reviewing briefly
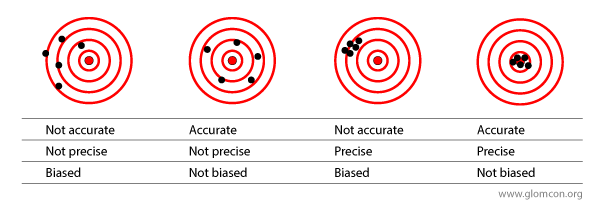
As reviewed by Botev et al. , several studies have aimed to assess the bias and precision of MDRD by comparing estimated GFRs to measured GFRs.
In sum, the mean difference between estimated GFR and measured GFR was anywhere between -29 to +12 ml/min/1.73m2 with a substantial bias toward underestimating the actual GFR at higher values and designating patients as having kidney disease when they do not have any disease. In lower GFR values (somewhere below 20 ml/min) the formula is wrong in the opposite direction, which is overestimating the actual GFR.
Nevertheless, systematic bias (targets 1 and 3 in the above image) is not the only issue here. A lack of precision (targets 1 and 2 in the above image) further erodes the ability to predict the actual GFR in a given individual. At best, one would need to accept a 30% margin of error in both directions to capture 89% of estimates. At worst, nearly half (47%) of estimates fall outside of this +/- 30% margin of error (e.g., when actual GFR is 50 ml/min, 47% of estimates are somewhere below 35 ml or above 65 ml/min).
While CKD-EPI provides a less biased estimate, the precision of any given estimate has only marginally improved (MDRD more like target 1 and CKD-EPI more like target 2).
Here a schematic illustration of the imprecision of estimating GFR using the MDRD formula based on more than 5000 study participants (derived from a study by Stevens et al. ):
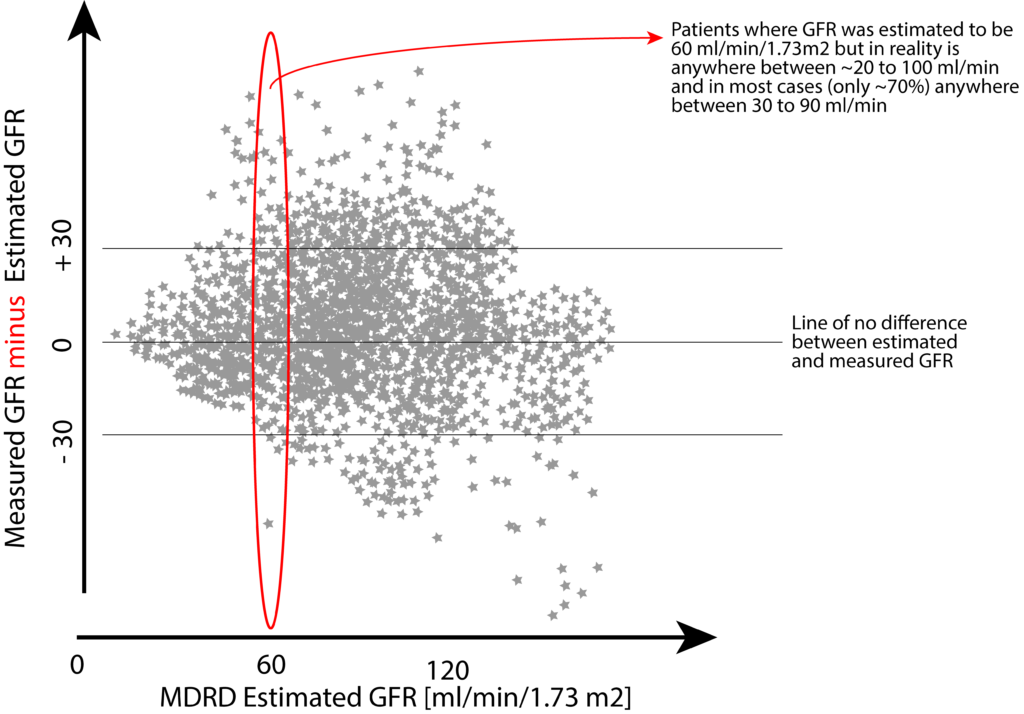
Further complicating is the fact that two individuals of the same sex, same serum creatinine, same weight, and height, may have different GFRs when directly measured. To this date, the exact reason for this difference is not fully understood, but in general, assumed to be due to muscle mass.
Studies have looked at the use of the MDRD formula in different ethnicities, such as the African American population or Chinese population. What we have learned from these studies:
- MDRD underestimates the actual GFR in a substantial number of white people
- MDRD underestimates the actual GFR in a substantial number of people designated as African-Americans.
- The number of African-American patients misclassified as having low GFR is less than the number of non-African Americans that are misclassified as having low GFR (True GFR is underestimated in non-African Americans, but not so much in African Americans. (Because we are concerned about labeling African Americans as having CKD, but not so much concerned about this in the white population?)
- Because of points 1,2, and 3, we classify a substantial number of patients as having CKD who do not have any disease. But we miss-classify more whites than Blacks as having CKD
- Due to the imprecision, we also classify a substantial number in both populations as being healthy, while indeed they may have clinically significant compromise in their renal function (more African Americans than whites though).
- The introduction of a correction factor based on skin color does not improve the precision, but merely shifts the bias and decreases the number of patients misclassified as having CKD, while at the same time increasing the number of patients with actual renal disease who are now being mislabeled as healthy.
- The MDRD discriminates not between “African American” and “whites” but between “African American” and “non-African American.” Within the category of non-African Americans are all others, such as whites, Hispanic/Latinos, Asians, Middle Eastern, Native American, and others. However, significant differences in creatinine excretion rates among these populations do exist, which are due to social, nutrition, genetic, and environmental factors. For example, the MDRD formula does overestimate the actual GFR in a significant number of Chinese patients with advanced CKD.
To improve the “accuracy” (move population mean of estimated GFR closer to the population mean of measured GFR), the concept of ethnicity-based correction was introduced. However, a significant limitation got lost in the translation: the correction factor does not affect the lack of precision.
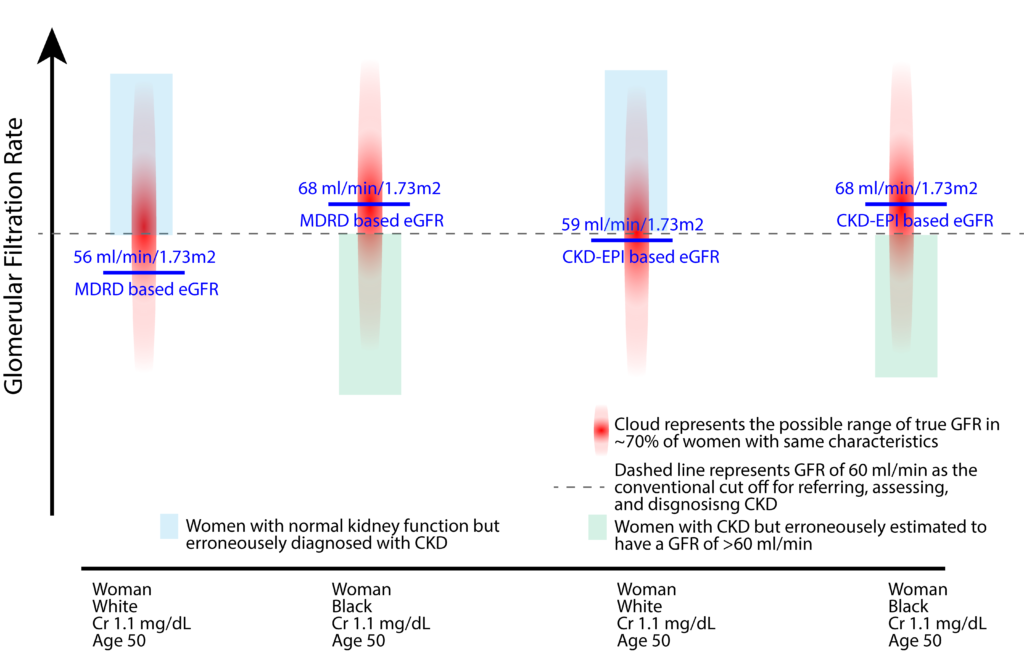
In the above example, we would diagnose a 50-year-old white woman with a serum creatinine of 1.1 mg/dL as having CKD using both MDRD or CKD-EPI formula. However, using these formulae, we accept that a substantial number of white women (blue box), who have a GFR >60 ml/min are erroneously categorized as having CKD. In this example, the MDRD and CKD-EPI formulae more sensitive in diagnosing CKD in white women.
For the Black women in this example, our diagnostic principles seem to have reversed. The formulae appear to be less sensitive around the diagnostic threshold for CKD. A substantial number of patients with a GFR<60 (green box) will not be recognized as having CKD due to the application of a skin-color-based adjustment factor.
There is the argument that if skin-color-based discrimination is removed, we would designate a large number of healthy self-identified African Americans or Black people as having CKD, and therefore cause significant emotional distress.
It is peculiar that we are willing to accept a lack of specificity for the sake of sensitivity and early diagnosis in non-African American patients, who are predominantly white. At the same time, we are concerned about emotional distress in Black patients, who, as a community, are at higher risk for CKD. This reverse standard makes this argument appear misguided at best, and racist at worst.
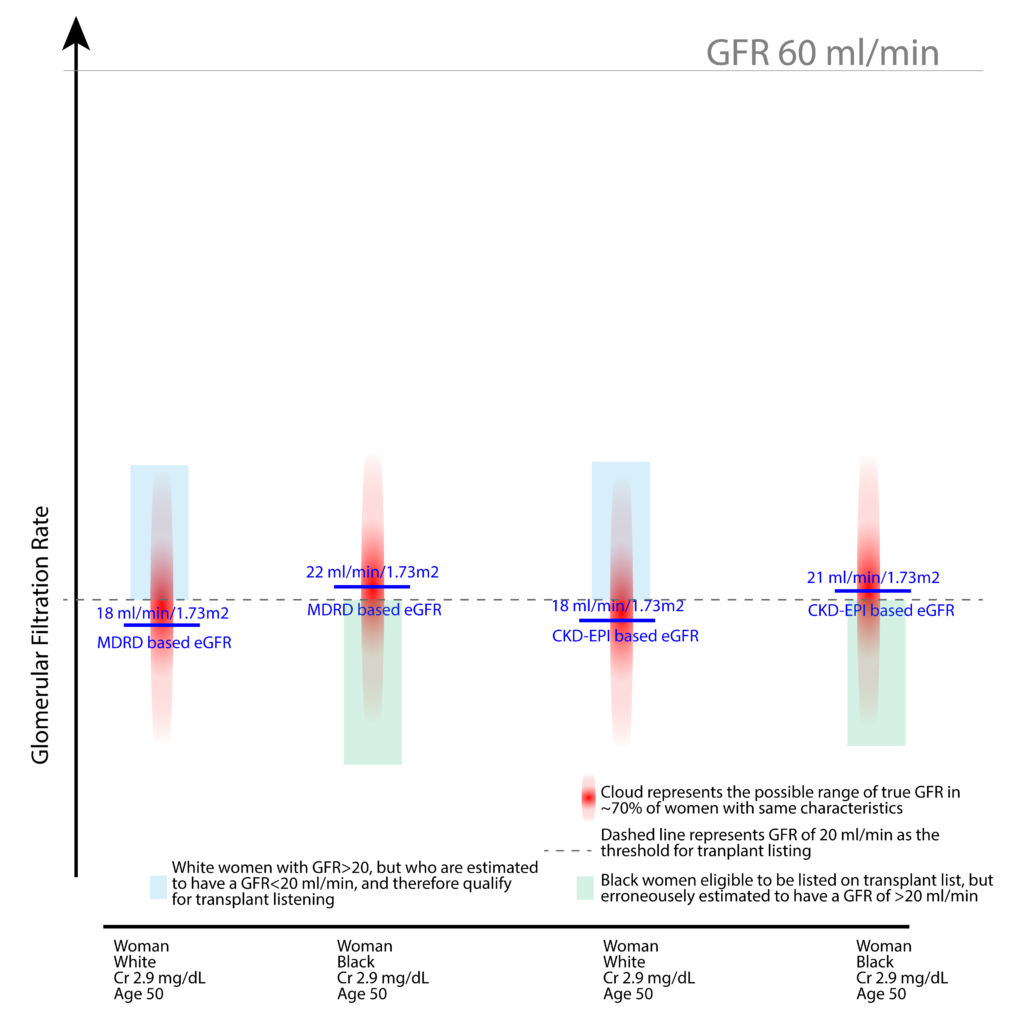
Similar discrimination can be observed when it comes to the eGFR threshold necessary for being placed on the transplant waiting list. Currently, we are willing to accept the listing of members of the majority population in the U.S. on to the transplant waiting list, even when their GFR has not reached the threshold of 20 ml/min (see below illustration).
Nevertheless, some argue that removal of skin-color-based discrimination would unnecessarily place too many patients identified as African American or Black on the transplant waiting list when their GFR has not reached the threshold of 20 ml/min.
The removal of the skin-color-based discrimination will indeed allow for the listening of a more significant proportion of African-American or Black patients with GFR of >20 ml/min than it currently does for non-African Americans. However, this should be a welcome correction, given how disproportionately this community is affected and the barriers they encounter in accessing care.
CKD Staging:
It is likely that when not using the adjustment factor as a threshold to screen and diagnose CKD, more patients who self-identify as African-American or who are thought to be African-American will be erroneously diagnosed as having CKD when indeed there is no evidence of kidney disease. The limitation of our current eGFR based CKD staging has been criticized for quite some time and well summarized by Glassock and Winearls.
A substantial proportion of our population (Black, White, Asian, Hispanic, Native-American, and all others) are currently erroneously being classified as CKD stage 3 without any evidence of kidney disease whatsoever. On the other hand, experience teaches that those particularly vulnerable to poor long-term outcomes are frequently overlooked as having kidney disease, perhaps also due to an “up correction” of the eGFR and a GFR centric approach to renal disease diagnosis.
The imprecision of eGFR:
Porrini and colleagues provided a more in-depth assessment of the imprecision of estimating GFR formulae based on serum creatinine or cystain C in Nature Reviews Nephrology.
This review also provides answers to those who wonder whether cystatin C based GFR estimates should replace creatinine-based eGFR. The answer is complicated. In the review referenced, the authors note “…we consider that the extremely wide margins of error for both creatinine-based and cystatin C- based estimation of GFR make it impossible to prove that these values can be corrected by mathematical modeling.”
Cystatin based GFR estimates do not have an ethnicity bias that creatinine-based eGFR has, and therefore do not require the use of an adjustment factor based on ancestry or skin color. However, they remain unacceptably imprecise in predicting an individual’s GFR. Cystatin C based GFR estimates may be a step toward the right direction (moving from bias and imprecision to just imprecision), but it is not a solution to our current practice, that is using an estimate with wide margins of error, to make a diagnosis and lifechanging decisions. Estimated GFR should be understood as what it is, that is an ESTIMATE, which will NOT become more precise by incorporating ethnicity into the calculation.
The use of current estimating GFR calculations (creatinine or cystatin C based) for disease classification suggests precision and accuracy, which they do not have. The answer to this dilemma should be a nuanced approach to our diagnosis and management of CKD until we have better markers of disease state in individual patients.
As Glassock and Winearls put it, “GFR can be estimated, but diagnosis cannot. Proper treatment requires precision in diagnosis.”
Or as Porrini et al. note in their response to Levey et al.’s correspondence:
“…the average error of eGFR equations is as wide as ±30%, or even larger. However, methods of evaluating formulas that estimate GFR must be adapted to the needs of patients and not to the evident limitations of eGFR equations. The error in eGFR that we tolerate in nephrology would be unacceptable in other areas of medicine. How many patients with hypertension would be diagnosed as normotensive (or vice versa) if we used a sphygmomanometer with an error of 30–50%?”
What would be then the solution?
Range and Trend
For the most part, nephrologists practice intuitively based on the eGFR range and trend over time. We know that GFR (and with that eGFR) can physiologically fluctuate throughout the day and on day by day basis without suggesting a disease progression. What matters more than a single value, are long term trend over months to years and the slope of GFR decline. Most clinicians also use proteinuria and other risk factors to make diagnostic, prognostic, and treatment decisions – for the most part, unless deciding referral to a nephrologist, dose adjusting drugs, or placing on the transplant waiting list. Then suddenly, non-nephrologists and nephrologist alike apply eGFR as if it is a precise estimate that can be used in dichotomous decision trees. But it cannot and should not.
We need to bring range and trend explicitly into the clinical practice and instead of providing a single value, be more transparent about the scientific uncertainty of the calculated estimate and provide the clinician a “reasonable” range. This range can be a two-standard-deviations interval, or more practically, we can use the current two possible values (CKD-EPI eGFR with and without ethnicity adjustment) as the upper and lower bounds of an estimate. Clinicians can use the lower number to be on the alert for early transplant referral, yet use the higher value to consider drug dosing and the possible need for 24-hour creatinine clearance measurement for a more individualized estimate.
In conclusion:
As the Black community is disproportionately suffering from kidney disease, access to care, and adverse outcomes, we should embrace strategies to heighten the sensitivity and enable early recognition, rather an effort to improve population statistics at the cost of individual patients.
These biases are some of the significant points of criticism around using the social construct “race” that is currently based on an ancestral connection to Africa or skin color to discriminate in medicine.
The random, subjective, and discriminatory determination is further highlighted when considering the diversity of the Black community.
As a final note, it should be added that the researchers who devised a special formula for the minority population likely did so with all the best intentions. The attempt to tailor medicine to individual subgroups of the population, rather have the characteristics and needs of the majority dominate all corners of society is a noble cause.
However, when doing so, we must assess and consider the impact carefully.
A proposed set of criteria for the introduction of ethnicity-based clinical decision making should fulfill the following needs:
- ETHNIC IDENTITY: We must identifying the subpopulation in a fair, consistent, accurate, scientific, and socially acceptable manner
- HEALTH BENEFIT: The “algorithm” must improve the care for members of the target subpopulation.
- NEGATIVE IMPACT: What proportion of the target population will benefit, and what proportion will be negatively impacted? Before the broad adoption of ethnicity or ancestry based medical care, the evidence must be demonstrated about the positive and negative impact on individuals within the target community. A thorough assessment, transparency, and disclosure must be part of a rigorous evaluation before any broad implementation.
- EQUITABLE AMONG ALL: The “algorithm” must create or further contribute toward an equitable balance between all subgroups of the society.
- SELF GOVERNMENT: The “algorithm” can only be incorporated into clinical practice through the active involvement of members of the target sub-population. It must be broadly vetted and endorsed by the subpopulation before being “institutionalized.”
In the case of skin-color-based discrimination of GFR estimation, none of the above-stated imperatives apply.
It is time to do away with this well-intended discrimination until we have better ways of predicting GFR in individuals of all ethnic backgrounds.
For further reading:
Reconsidering the Consequences of Using Race to Estimate Kidney Function
Precision in GFR Reporting. Let’s Stop Playing the Race Card
Estimated GFR: time for a critical appraisal
Hidden in Plain Sight — Reconsidering the Use of Race Correction in Clinical Algorithms
Using race in the estimation of glomerular filtration rates: time for a reversal?
Screening for CKD with eGFR: Doubts and Dangers
Estimation of Glomerular Filtration Rate With vs Without Including Patient Race

Interesting!
see also our articles (and letter) on the same topic
https://pubmed.ncbi.nlm.nih.gov/30910379/
https://pubmed.ncbi.nlm.nih.gov/21441133/
https://pubmed.ncbi.nlm.nih.gov/32702094/
Thank you for the great references! I will incorporate this important work in the next version of this perspective.
[…] Shortcomings of the current GFR estimating equations […]